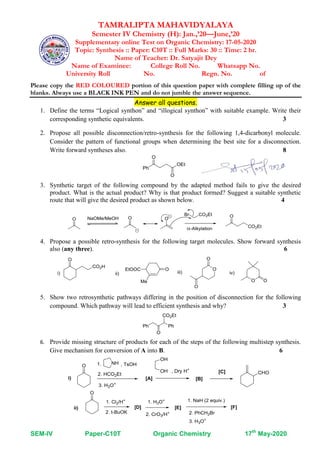
Question paper and its solution of supplementary on line test on synthesis
- 1. SEM-IV Paper-C10T Organic Chemistry 17th May-2020 TAMRALIPTA MAHAVIDYALAYA Semester IV Chemistry (H): Jan.,’20—June,’20 Supplementary online Test on Organic Chemistry: 17-05-2020 Topic: Synthesis :: Paper: C10T :: Full Marks: 30 :: Time: 2 hr. Name of Teacher: Dr. Satyajit Dey Name of Examinee: College Roll No. Whatsapp No. University Roll No. Regn. No. of Please copy the RED COLOURED portion of this question paper with complete filling up of the blanks. Always use a BLACK INK PEN and do not jumble the answer sequence. Answer all questions. 1. Define the terms “Logical synthon” and “illogical synthon” with suitable example. Write their corresponding synthetic equivalents. 3 2. Propose all possible disconnection/retro-synthesis for the following 1,4-dicarbonyl molecule. Consider the pattern of functional groups when determining the best site for a disconnection. Write forward syntheses also. 8 3. Synthetic target of the following compound by the adapted method fails to give the desired product. What is the actual product? Why is that product formed? Suggest a suitable synthetic route that will give the desired product as shown below. 4 4. Propose a possible retro-synthesis for the following target molecules. Show forward synthesis also (any three). 6 5. Show two retrosynthetic pathways differing in the position of disconnection for the following compound. Which pathway will lead to efficient synthesis and why? 3 6. Provide missing structure of products for each of the steps of the following multistep synthesis. Give mechanism for conversion of A into B. 6
- 2. ___________________________________________________________________________ SEM-IV Paper-C10T Organic Chemistry 17th May-2020 TAMRALIPTA MAHAVIDYALAYA Semester IV Chemistry (H): Jan.,’20—June,’20 Supplementary online Test on Organic Chemistry: 17-05-2020 Topic: Synthesis :: Paper: C10T :: Full Marks: 30 :: Time: 2 hr. Name of Teacher: Dr. Satyajit Dey Solution to Supplementary online Test on Organic Chemistry 1. Define the terms “Logical synthon” and “illogical synthon” with suitable example. Write their corresponding synthetic equivalents. 3 Answer: Definition of logical synthon and illogical synthon: If, disconnection of a bond in a target molecule leads to a synthon which has natural polarity that can be demonstrated from the latent polarity of the molecule, such synthons are called logical synthons or natural synthons. Disconnection of a bond in a target molecule sometimes leads to a synthon which has polarity that is reversed to its normal latent polarity. Such synthons are called illogical synthons or unnatural synthons. According to the concept of latent polarity, the polarity of the carbon atom next to the hydroxyl group bearing carbon should be negative. But, disconnection of γ-hydroxy carbonyl compound leads to a synthon which has positive polarity on that carbon. Therefore, this synthon is illogical synthon. However, the α-carbon with respect to carbonyl group should be negative, and the disconnection leads to a carbonyl synthon that acquires the same polarity as exhibited by latent polarity. Therefore, this synthon is logical synthon. 2. Propose all possible disconnection/retro-synthesis for the following 1,4-dicarbonyl molecule. Consider the pattern of functional groups when determining the best site for a disconnection. Write forward syntheses also. 8
- 3. ___________________________________________________________________________ SEM-IV Paper-C10T Organic Chemistry 17th May-2020 Answer: Write forward synthesis accordingly. 3. Synthetic target of the following compound by the adapted method fails to give the desired product. What is the actual product? Why is that product formed? Suggest a suitable synthetic route that will give the desired product as shown below. 4 Answer: Synthetic target by the given method fails to give the desired product. This is because the α-protons of α-bromo ethyl acetate is more acidic than the α-protons of acetone due to resonance stabilization by ester carbonyl as well as electron withdrawing inductive effect of bromine atom. Consequently, the carbanion derived from acetone undergoes proton exchange with α-bromo ethyl acetate faster than α- alkylation reaction which could provide the desired product. The new carbanion resulting from α-bromo ethyl acetate reacts with acetone to undergo Darzen’s reaction as depicted below.
- 4. ___________________________________________________________________________ SEM-IV Paper-C10T Organic Chemistry 17th May-2020 To get the desired product, either pre-formed enolate can be used or activating group at -position of acetone can be introduced to increase its acidity compared to bromo ethyl acetate. Using pre-formed enolate: Introducing Activation group: 4. Propose a possible retro-synthesis for the following target molecules. Show forward synthesis also (any three). 6 i) Retro-synthesis: Synthesis:
- 5. ___________________________________________________________________________ SEM-IV Paper-C10T Organic Chemistry 17th May-2020 ii) Retro-synthesis: Synthesis: iii) Retro-synthesis: Synthesis:
- 6. ___________________________________________________________________________ SEM-IV Paper-C10T Organic Chemistry 17th May-2020 iv) Retro-synthesis: Synthesis: 5. Show two retrosynthetic pathways differing in the position of disconnection for the following compound. Which pathway will lead to efficient synthesis and why? 3 Answer: Proposed Retro-synthetic Paths: The path “a” would lead to the efficient synthesis because: i) path “a” involves only a single substrate, ethyl phenylacetate, which is inexpensive and readily available; ii) path “a” demonstrates to a single step synthesis; iii) dibenzyl ketone, one of the starting materials of retro-synthetic path “b” would probably have to be synthesized in a three-step synthesis involving Grignard reaction. So, path “a” would lead to a single step synthesis, whereas path “b” would lead to a three-step synthesis.
- 7. ___________________________________________________________________________ SEM-IV Paper-C10T Organic Chemistry 17th May-2020 6. Provide missing structure of products for each of the steps of the following multistep synthesis. Give mechanism for conversion of A into B. 6 Answer:
