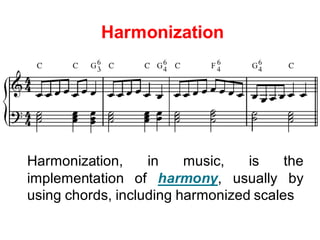
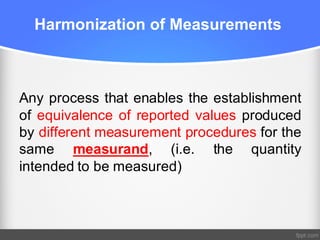
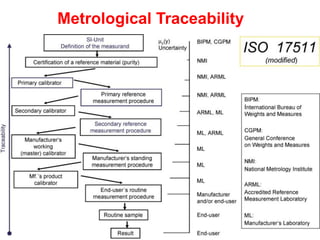
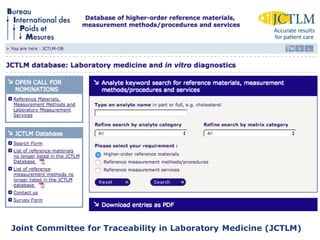
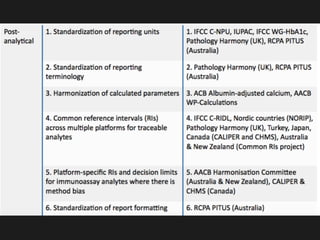
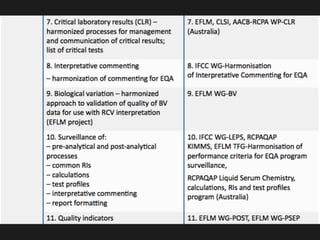
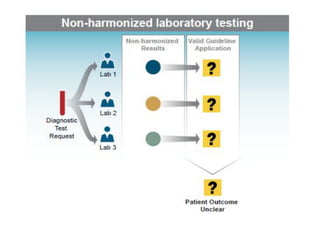
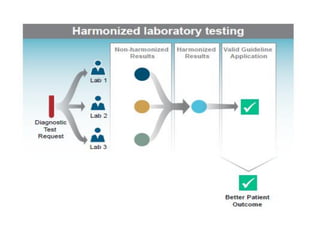
1) Harmonization of laboratory testing aims to make test results from different laboratories comparable by adjusting for differences in measurement procedures and methods.
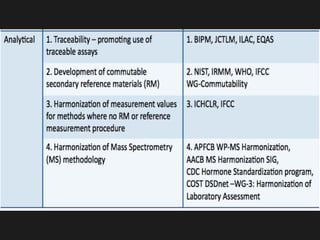
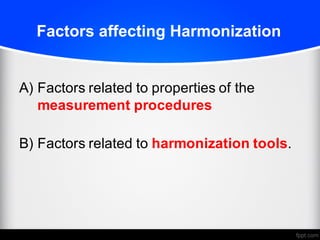
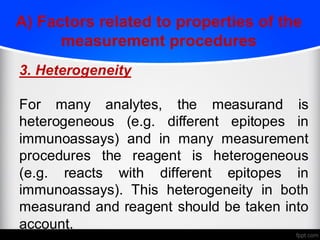
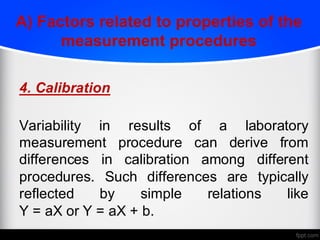
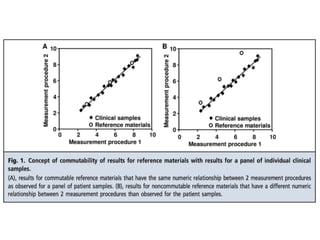
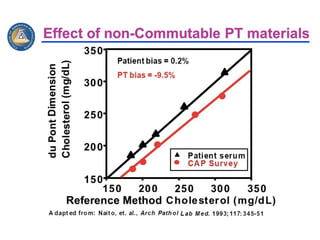
2) Factors like reproducibility, linearity, heterogeneity, calibration, commutability, stability, sustainability, and value assignment of measurement procedures and reference materials can affect the possibility and success of harmonization efforts.
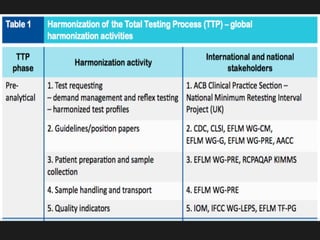
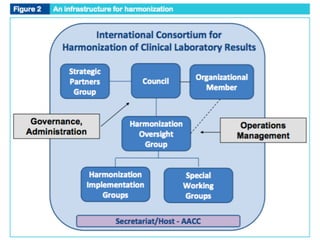
3) Global initiatives are working to prioritize measurands, coordinate harmonization activities, and promote the standardization and statistical harmonization of test results.