9. Coordination compounds class 12.pptx
- 1. CLASS 12, CHEMISTRY, CHAPTER NO. 9 COORDINATION COMPOUNDS BY: MEGHA SOLANKI, PGT- CHEMISTRY, KV MANKHURD, MUMBAI
- 2. REVISED SYLLABUS FOR 2020-21 • Coordination compounds - Introduction, • ligands, • coordination number, • colour. • Magnetic properties and shapes. • IUPAC nomenclature of mononuclear coordination compounds. • Bonding - Werner's theory, VBT, and CFT.
- 3. COORDINATION COMPOUDS IN OUR DAILY LIFE • Earlier, these compounds were called as complex compounds. • Chlorophyll, haemoglobin and vitamin B12 are coordination compounds of magnesium, iron and cobalt respectively. • Variety of metallurgical processes, industrial catalysts and analytical reagents involve the use of coordination compounds. • Coordination compounds also find many applications in electroplating, textile dyeing and medicinal chemistry. vitamin B12 haemoglobin
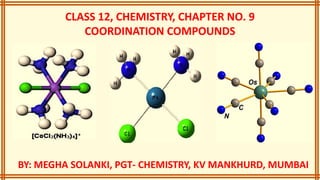
- 4. COORDINATION COMPOUNDS DISCOVERY • The sustained and systematic development of modern coordination chemistry began with the discovery by the French chemist B.M. Tassaert in 1798 that ammoniacal solutions of cobalt chloride (CoCl3) develops a brownish- red colour. • Further experiments were done by other scientists on this solutions and it was found that it was a mixture of various coloured compounds of Co, Cl and NH3. • These compounds were separated and it was found that in these compounds, the no. of Co and Cl atoms remain same but no. of NH3 molecules changes. And simultaneously the colour of the compounds change. • When 1 mole of each of these compounds was treated with excess AgNO3 solution, different no. of moles of AgCl were precipitated as shown in the table below.
- 5. The above results can be explained if we consider the following chemical structure of the above compounds:
- 6. • It is clear from the structural chemical formula of these compounds that only the ions present outside the square brackets can react with AgNO3 and they are called primary valence. • All the atoms within square brackets behave like a single ion. • Werner proposed the term secondary valence for the number of groups bound directly to the metal ion; in each of these examples the secondary valences are six. • Note that the last two compounds in Table 9.1 have identical empirical formula, CoCl3.4NH3, but distinct properties. Such compounds are termed as isomers.
- 7. WERNER’S THEORY OF COORDINATION COMPOUNDS • Alfred Werner (1866-1919), a Swiss chemist was the first to formulate his ideas about the structures of coordination compounds. Werner in 1898 gave his theory of coordination compounds. The main postulates are: 1. In coordination compounds, metals show two types of linkages (valences)-primary and secondary. 2. The pri. valences are normally ionisable and are satisfied by -ve ions(counter ions) 3. The sec. valences are non ionisable. These are satisfied by neutral molecules or -ve ions. The sec. valence is equal to the coordination no. and is fixed for a metal. 4. The ions/groups bound by the sec. linkages to the metal have characteristic spatial arrangements( called polyhedra) corresponding to different coordination no. The metal along with secondarily linked groups are called coordination compounds. 5. octahedral, tetrahedral and square planar geometrical shapes are more common in coordination compounds of transition metals. Examples: [Co(NH3)6]3+, [CoCl(NH3)5]2+ and [CoCl2(NH3)4]+ are octahedral entities, while [Ni(CO)4] is tetrahedral and [PtCl4]2– is square planar.
- 10. NOW SOLVE THIS Example 9.1: On the basis of the following observations made with aqueous solutions, assign secondary valences to metals in the following compounds: ANSWERS: (i) 4. (ii) 6. (iii) 6. (iv) 6 (v) 4
- 11. DIFFERENCE BETWEEN A DOUBLE SALT AND A COMPLEX
- 12. DOUBLE SALT VS COORINATION COMPOUNDS
- 13. SOME EXAMPLES OF DOUBLE SALT potash Alum - K2SO4.Al2(SO4)3.24H2O Ammonium Alum - (NH4)2 Al2(SO4)3.24H2O Mohr’s Salt - FeSO4(NH4)2 SO4.6H2O Carnallite - KCl.MgCl2.6H2O,
- 14. SOME IMPORTANT TERMS PERTAINING TO COORDINATION COMPOUNDS (a) Coordination entity: A coordination entity constitutes a central metal atom or ion bonded to a fixed number of ions or molecules. For example, [CoCl3(NH3)3] is a coordination entity in which the cobalt ion is surrounded by 3 ammonia molecules and 3 chloride ions. Other examples are: [Ni(CO)4], [PtCl2(NH3)2], [Fe(CN)6]4–, [Co(NH3)6]3+. (b) Central atom/ion In a coordination entity, the atom/ion to which a fixed number of ions/groups are bound in a definite geometrical arrangement around it, is called the central atom or ion. For example, the central atom/ion in the coordination entities: [NiCl2(H2O)4], [CoCl(NH3)5]2+ and [Fe(CN)6]3– are Ni2+, Co3+ and Fe3+ respectively. These central atoms/ions are also referred to as Lewis acids.
- 15. (c) Ligand
- 16. Types of ligand There are basically three types of ligands: 1. Monodentate or Unidentate ligands 2. Polydentate ligand or multidentate ligand 3. Ambidentate ligand 4. Chelate ligand 1. Monodentate ligand
- 19. When a di- or polydentate ligand uses its two or more donor atoms to bind a single metal ion, it is said to be a chelate ligand. The number of such ligating groups is called the denticity of the ligand. Such complexes, called chelate complexes tend to be more stable than similar complexes containing unidentate ligands 4. Chelate ligand Ethylenediaminetetraacetate ion (EDTA4–) is an important hexadentate ligand. It can bind through two nitrogen and four oxygen atoms to a central metal ion. EDTA4- EDTA4- in chelation Ethylenediamine-metal chelate
- 20. (d)Coordination number(CN) It is important to note here that coordination number of the central atom/ion is determined only by the number of sigma bonds formed by the ligand with the central atom/ion. Pi bonds, if formed between the ligand and the central atom/ion, are not counted for this purpose.
- 21. (e) Coordination sphere The central atom/ion and the ligands attached to it are enclosed in square bracket and is collectively termed as the coordination sphere or inner sphere. The ionisable groups are written outside the bracket and are called counter ions or outer sphere or ionization sphere. For example, in the complex K4[Fe(CN)6], the coordination sphere is [Fe(CN)6]4– and the counter ion is K+.
- 22. • The spatial arrangement of the ligand atoms which are directly attached to the central atom/ion defines a coordination polyhedron about the central atom. • The most common coordination polyhedra are octahedral, square planar and tetrahedral. • For example, [Co(NH3)6]3+ is octahedral, [Ni(CO)4] is tetrahedral and [PtCl4]2– is square planar. (f) Coordination polyhedron Shapes of different coordination polyhedra
- 23. (g) Oxidation number of central atom
- 25. (g) Homoleptic and Heteroleptic ligands
- 35. Ligand names in complexes
- 36. LATIN NAMES OF SOME TRANSITION ELEMENT
- 39. ANSWER THESE
- 41. • Compounds that have the same chemical formula but different structural arrangements are called isomers. • In coordination compounds, due to their complicated formulae, the variety of bond types and the number of shapes possible, many different types of isomerism occur. ISOMERISM IN COORDINATION COMPOUNDS ***** Isomerism may be skipped as it is not in syllabus for 2020-21 *****
- 42. STEREO ISOMERISM They have same chemical formula and chemical bonds but they have different spatial arrangement STRUCTURAL ISOMERISM ISOMERISM Structural isomers have the same chemical formula but they have different bonds
- 44. STEREOISOMERISM: GEOMETRICAL ISOMERISM • This type of isomerism arises in heteroleptic complexes due to different possible geometric arrangements of the ligands. • It is mostly found in the complex compounds with coordination numbers 4 and 6 but not found in the compounds with CN = 2 and 3. It is because different geometrical arrangements of ligands are not possible around the central metal in these complexes. All 3 arrangements are similar to one- another in CN = 3 with trigonal planar geometry Both arrangements are similar to each- other for CN =2, with linear geometry • Geometrical isomerism is also not possible in tetrahedral complexes with CN = 4 because all the four ligands are adjacent or equidistant to one another in tetrahedral complex.
- 45. GEOMETRICAL ISOMERISM IS CIS-TRANS ISOMERISM
- 46. SQUARE PLANAR COMPLEX WITH FORMULA MA2B2
- 47. GEOMETRICAL ISOMERISM IN SQUARE PLANAR COMPLEX WITH FORMULA MA2BC They also exhibit 1 cis and 1 trans isomer Cis isomer –When similar ligands(AA) are adjacent. Trans isomer –When similar ligands are at opposite corners.
- 48. GEOMETRICAL ISOMERISM IN SQURE PLANAR COMPLEX WITH FORMULA MABCD They show three isomers-two cis and one trans. Here, we fix the position of one ligand and found its isomers for any another ligand. For example, in the following figures, a is cis with d two times and trans with d one time. More than 3 arrangements are not possible In the following example, Br has a fixed position. With any other ligand, it will remain cis two times at both the adjacent corners while it remains trans with every one only once when the ligand takes the opposite corner.
- 49. GEOMETRICAL ISOMERISM IN SQUARE PLANAR COMPLEX WITH UNSYMMETRICAL BIDENTATE LIGAND OF FORMULA M(AB)2 In this, when ligands connect to metal in same manner, they form cis-isomer and when connect in opposite manner they form trans isomer EXAMPLE: Square planner complexes with formula MA4 and MA3B have no geometrical isomers as only one arrangement is possible for them.
- 50. SUMMARY OF GEOMETRICAL ISOMERS IN SQUARE PLANAR COMPLEX
- 51. GEOMETRICAL ISOMERISM IN OCTAHEDRAL COMPLEXES • Octahedral complexes with formula MA4B2, MA4BC, MA3B3, MA3BCD, MA2B2C2 , MABCDEF, M(AA)2B2 , M(AA)(BB)C2, M(AA)(BB)(CC) M(AA)(BB)CD etc. show geometrical isomerism but MA6 and MA5B do not. Here, AA, BB etc. are bidentate ligands. • Here we will study the geometrical isomers of some of the above octahedral complexes
- 52. For MA4B2, the B atoms can be cis or trans to each other. GEOMETRICAL ISOMERS OF OCTAHEDRAL COMLEXES WITH FORMULA MA4B2
- 53. GEOMETRICAL ISOMERS OF OCTAHEDRAL COMLEXES WITH FORMULA MA3B3 • The geometric isomers of these complexes are not called as cis-trans isomers but are called as Facial (FAC) and Meridional (MER) isomers. • If three donor atoms of the same ligands occupy adjacent positions at the corners of an octahedral face, we have the facial (fac) isomer. • When the positions are around the meridian of the octahedron, we get the meridional (mer) isomer.
- 54. GEOMETRICAL ISOMERS OF OCTAHEDRAL COMLEXES WITH FORMULA M(AA)2B2 These complexes also show cis-trans isomerism
- 55. SUMMARY OF GEOMETRICAL ISOMERS IN OCTAHEDRAL COMPLEXES
- 56. • The two forms are called dextro (d) and laevo (l) depending upon the direction they rotate the plane of polarised light in a polarimeter (d rotates to the right, l to the left) SREREOISOMERISM : OPTICAL ISOMERISM • Optical isomers are mirror images that cannot be superimposed on one another. These are called as enantiomers. • The molecules or ions that cannot be superimposed are called chiral. They don’t have plane of symmetry. • Optical isomerism is common in octahedral complexes involving didentate ligands.
- 57. Some examples of optical isomers: Optical isomers of octahedral compounds with formula M(AA)3
- 58. Optical isomers of octahedral complexes with formula M(AA)2B2
- 59. ANSWER THE FOLLOWING: Trans has a plane of symmetry hence it will not give optical isomers.
- 60. STRUCTURAL ISOMERISM:- (1) LINKAGE ISOMERISM • Linkage isomerism arises in a coordination compound containing ambidentate ligand. • When different atoms of the same ligand are boded or linked to metal, then they give rise to linkage isomers as shown below: • Other ligands capable of switching donor atom are SCN-, CN- etc.
- 61. STRUCTURAL ISOMERISM:- (2) COORDINATION ISOMERISM • This type of isomerism arises from the interchange of ligands between cationic and anionic entities of different metal ions present in a complex. • An example is provided by [Co(NH3)6][Cr(CN)6], in which the NH3 ligands are bound to Co3+ and the CN– ligands to Cr3+ .In its coordination isomer [Cr(NH3)6][Co(CN)6], the NH3 ligands are bound to Cr3+ and the CN– ligands to Co3+ .
- 62. • This form of isomerism arises when the counter ion in a complex salt is itself a potential ligand and can displace a ligand which can then become the counter ion. • An example is provided by the following ionisation isomers [Co(NH3)5 (SO4)]Br and [Co(NH3)5Br]SO4. • These isomers give different ions in solutions. • In the above example, the first one releases Br- ions and can give precipitation of AgBr with AgNO3. • The second one releases SO4 2- ions which can give precipitation of BaSO4 with BaCl2 solution. STRUCTURAL ISOMERISM:- (3) IONISATION ISOMERISM
- 63. STRUCTURAL ISOMERISM:- (4) SOLVATE ISOMERISM • This form of isomerism is known as ‘hydrate isomerism’ in case where water is involved as a solvent. • This is similar to ionisation isomerism. • Solvate isomers differ by whether or not a solvent molecule is directly bonded to the metal ion or merely present as free solvent molecules in the crystal lattice. • An example is provided by the aqua complex [Cr(H2O)6]Cl3 (violet) and its solvate isomer [Cr(H2O)5Cl]Cl2.H2O (grey-green). • Another example is given below:
- 64. EFFECTIVE ATOMIC NUMBER RULE • Effective Atomic Number Rule is proposed by Sidgwick. The total number of electrons possessed by central transition metal ion after the donation of electrons by the ligand is called as an Effective Atomic Number(EAN) • A complex is stable if the effective atomic number is equal to the atomic number of nearest inert gas. • Example: Calculate the effective atomic number of the following complexes: (1) K4[Fe(CN)6] (2) [Co(NH3)]Cl3 1. K4[Fe(CN)6] Number of electrons in Fe2+ = 24 Number of electrons by Six CN = 2×6 = 12 Total number of electrons possessed by Fe2+ = 24 + 12 Therefore, the effective atomic number = 36. 2. [Co(NH3)]Cl3 Number of electrons in Co+3 = 24 Number of electrons by Six NH3 = 2×6 = 12 Total number of electrons possessed by Co+3 = 24 + 12 Therefore, the effective atomic number = 36. BONDING IN COORDINATION COMPOUNDS: WHY ARE THEY FORMED
- 65. TABLE OF EAN OF SOME COMPLEXES
- 66. Werner’s Theory was the first to describe the bonding in coordination compounds. But his theory was not satisfactory. Drawbacks of Werner’s theory: This theory failed to answer basic questions like: (i) Why only certain elements possess the remarkable property of forming coordination compounds? (ii) Why the bonds in coordination compounds have directional properties? (iii) Why coordination compounds have characteristic magnetic and optical properties? BONDING IN COORDINATION COMPOUNDS
- 67. Many approaches have been put forth to explain the nature of bonding in coordination compounds viz. (i) Valence Bond Theory (VBT), (ii)Crystal Field Theory (CFT), (iii)Ligand Field Theory (LFT) (iv)Molecular Orbital Theory (MOT). Here, we shall focus mainly on VBT and CFT OTHER THEORIES TO EXPLAIN BONDING IN COORDINATION COMPOUNDS
- 68. VALANCE BOND THEORY This theory was given by Linus Pauling. According to this theory: • The metal atom or ion under the influence of ligands can use its (n-1)d, ns, np or ns, np, nd orbitals for hybridisation to yield a set of equivalent orbitals of definite geometry such as octahedral, tetrahedral, square planar and so on as shown in the following table: • These hybridised orbitals are allowed to overlap with ligand orbitals that can donate electron pairs for bonding. Such a bond is called coordinate covalent bond.
- 69. • These complexes can be of two types, depending upon the nature of ligand: (a) Low spin complexes: These are formed when ligand is strong field ligand like NH3, CN- etc. These ligands push the unpaired electrons of (n-1)d orbitals inside to be paired up. This process is called spin pairing. Thus metal gets one or two (n-1)d orbitals vacant which take part in hybridization. Due to pairing of electrons, such compounds are generally diamagnetic. They are also called as inner sphere or inner orbital or spin paired complexes. Examples are: dsp2 hybridization and d2sp3 hybridization.
- 70. (b) High spin complexes: These are formed when ligand is weak field ligand like F-, Cl- etc. These ligands can not push the unpaired electrons of (n-1)d orbitals to be paired up. That’s why metal uses one or two nd vacant orbitals for hybridization. As pairing of (n-1)d electron could not be done, unpaired electrons are present and hence these complexes are paramagnetic. They are also called as outer sphere or outer orbitals or spin free complexes. Examples are: sp3d and sp3d2 hybridization. • Spin pairing is not done in metal ions with d1, d2 and d3 configuration in penultimate shell as already vacant orbitals are available. • The total number of metal orbitals undergone hybridization is equal to its coordination no.(CN).
- 71. SOME MORE EXAMPLES OF COMPLEXES EXPLAINED THROUGH VBT
- 75. TABLE OF SOME COMPLEXES WITH THEIR DETAILS
- 76. NOW SOLVE THIS
- 77. • The series of increasing order of field strength of ligands is known as Spectrochemical series. • It is given as below: I– < Br– < SCN– < Cl– < S-2 < F– < OH– < C2O4 -2 < H2O < NCS− < (EDTA)-4 < NH3 < en < CN- < CO SPECTRO CHEMICAL SERIES
- 78. 1. The complex in which central transition metal ion has unpaired electrons is Paramagnetic. 2. The complex in which central transition metal ion has no unpaired electrons is diamagnetic. 3. The magnetic moment of a complex is calculated by the spin only formula M = √[n(n+2)] BM BM = Bohr Magneton The magnetic moment of complex compounds depends upon: • Type of hybridization. • The oxidation state of central transition metal ion. • The number of unpaired electrons. MAGNETIC PROPERTIES OF COORDINATION COMPOUNDS
- 79. MAGNETIC PROPERTIES OF COORDINATION COMPOUNDS:- EXPERIMENTAL RESULTS AND THEIR EXPLANATION BY VBT • The magnetic moment of coordination compounds can be measured by the magnetic susceptibility experiments. The results are discussed below: RESULT / OBSERVATIONS EXPLANATION BY VBT For metal ions with up to three electrons in the d orbitals, like Ti3+ (d1); V3+ (d2); Cr3+ (d3); the magnetic behaviour of these free ions and their coordination entities is similar. two vacant d orbitals are available for octahedral hybridisation with 4s and 4p orbitals. So no pairing is required. [Mn(CN)6]3– has magnetic moment of two unpaired electrons while [MnCl6]3– has a paramagnetic moment of four unpaired electrons. [Fe(CN)6]3– has magnetic moment of a single unpaired electron while [FeF6]3– has a paramagnetic moment of five unpaired electrons. [CoF6]3– is paramagnetic with four unpaired electrons while [Co(C2O4)3]3– is diamagnetic. This apparent anomaly is explained by valence bond theory in terms of formation of inner orbital and outer orbital coordination entities. [Mn(CN)6]3– [Fe(CN)6]3– and [Co(C2O4)3]3– are inner orbital complexes involving d2sp3 hybridisation, the former two complexes are paramagnetic and the latter diamagnetic. On the other hand, [MnCl6]3– , [FeF6]3– and [CoF6 -]3– are outer orbital complexes involving sp3d2 hybridisation and are paramagnetic corresponding to four, five and four unpaired electrons.
- 80. While the VB theory, to a larger extent, explains the formation, structures and magnetic behaviour of coordination compounds, it has following shortcomings: (i) It involves a number of assumptions. (ii) It does not give quantitative interpretation of magnetic data. (iii) It does not explain the colour exhibited by coordination compounds. (iv) It does not give a quantitative interpretation of the thermodynamic or kinetic stabilities of coordination compounds. (v) It does not make exact predictions regarding the tetrahedral and square planar structures of 4-coordinate complexes. (vi) It does not distinguish between weak and strong ligands. DRAWBACKS OF VBT
- 81. The crystal Field Theory was proposed by Hans Bethe and Van Vleck. This theory gives much more satisfactory explanation for the bonding and the properties of complex compounds than VBT. CRYSTAL FIELD THEORY
- 82. 1. The interaction between metal ion and the ligands are purely electrostatic. It means metal-ligand bond is 100% ionic in nature. 2. Negative ligands are treated as point charges. While the neutral ligands are treated as dipoles. Thus, the bonding in the complex may be an ion-ion interaction or an ion-dipole interaction. ASSUMPTIONS OF CRYSTAL FIELD THEORY
- 83. 3. The five d-orbitals in an isolated gaseous metal atom or ion are degenerate. Means they all have the same energy.
- 84. 4. When ligands approach to metal ion to form a complex, the electrons in the d- orbitals of the metal ion will be repelled by the negative charge or the lone pair electrons of the ligands due to the repulsion between the like charges. ligands approach to metal
- 85. 5. As a result the energy of the d- orbitals increases and the d-orbitals no longer remain degenerate. It results in the splitting of d- orbitals. energy of the d-orbitals increases Ligands approaches, repulsion occurs.
- 86. 6. the pattern of splitting of the d-orbitals depends on the number of ligands and their arrangement around the central metal atom or ion.
- 87. APPLICATION OF CFT IN OCTAHEDRAL COMPLEXES 1. In octahedral complexes, the metal ion is in the centre of the octahedral and the six ligands lie at the six corners of the octahedral along the three axes x, y and z.
- 88. 2. The approach of the ligands towards the central metal ion is considered a two step process: (a) In the first step the ligands approach the metal spherically. i.e. at the same distance from all the five d-orbitals. At this stage, the energy of all the d- orbitals is raised by the same amount i.e. the five d- orbitals still remain degenerate. At this stage whatever is the value of energy of d-orbitals is considered zero and it is called Barry centre.
- 89. (b) However, as the ligands come closer the dx2-y2, and dz2 orbitals experience more repulsion as they lie on axes and ligands are also approaching in the direction of axes and hence their energy is raised up from Barry centre. The set of these two orbitals with higher energy is called as eg orbitals. But rest of the three d- orbitals, dxy, dyz and dzx , which lie in between the axes experience less repulsion and hence their energy is lowered as compared to Barry centre. These three orbitals are known as T2g orbitals. This is called Crystal Field Splitting. (the names of these orbitals come from Mulliken symbols. T stand for triple degenerate orbital and e stand for double degenerate orbital. Subscript g (origin: German word gerade) means – symmetric with respect to inversion center. Subscript 2 – anti- symmetric with respect to perpendicular C2 axis.)
- 90. 3. The extent of splitting in energies is denoted by symbol ∆o where O represents Octahedral geometry. 4. Due to this splitting, the energy of two eg orbitals will increase by 3/5th of ∆o while that of three t2g orbitals will decrease by 2/5th of ∆o. 5. The magnitude of crystal field splitting depends upon the field strength of the ligand and the charge on the metal ion. 6. Strong field ligands cause large splitting whereas weak field ligands cause small splitting.
- 91. The first three electrons will occupy low energy t2g orbitals according to Aufbau principle and Hund’s rule. But the fourth electron have two paths to follow: (a) If the value of ∆o is lesser than pairing energy(in case of splitting by weak field ligands), then the fourth electron will not be paired but will occupy one of the eg orbitals. Thus we get a high spin complex with weak field ligands. (b) If the value of ∆o is greater than pairing energy(in case of splitting by strong field ligands), then the fourth electron will be paired up in t2g and will not move in the higher energy eg orbitals. Thus we get a low spin complex with strong field ligands. Assigning electrons in the d-orbitals of the metal ion in the octahedral coordination entities
- 92. It occurs in following steps : 1. Metal remains at the centre of tetrahedron. 2. Ligands approaches spherically towards the metal and the energy of all the five d- orbitals is raised equally due to repulsion between ligand electrons and d- orbital electrons of metal. 3. Crystal field splitting- In tetrahedral symmetry, the ligands approach to central metal not along the x, y, z axes but they lie in between them. Therefore the d- orbitals which lie in between the axes like dxy, dyz and dzx experience more repulsion than dx2- y2 and dz2. Thus dxy, dyz and dzx make higher energy level t2g whereas the remaining two make lower enery level called eg. APPLICATION OF CFT IN TETRAHEDRAL COMPLEXES
- 93. 6. Due to smaller value of ∆t, pairing energy is always greater than ∆t. Therefore the third electron choses to go in higher t2g orbitals instead of being paired. Hence low spin tetrahedral complexes are rarely observed. 4. Thus tetrahedral splitting is reversed of octahedral complexes. 5. The crystal field splitting energy(CFSE) in tetrahedral complexes is denoted by ∆t and it is smaller than CFSE in octahedral complexes. ( ∆t = 4/9 ∆o).
- 94. • for example [Ti(H2O)6 ]+3 absorbs wavelengths mostly in blue green portion of visible light (𝝀= 498 nm). The transmitted light does not have blue green light. Therefore, the remaining wavelengths together generate a pinkish purple colour. COLOURS IN COORDINATION COMPOUNDS • Transition metal atoms and their ions with one or more unpaired electrons and their complexes exhibit colours in both solid state and their solutions. • How a substance gets its colour: When white light passes through a substance, it absorbs some of the wavelengths. The light transmitted by the substances is deprived of the wavelengths that have been absorbed. If absorption occurs in the visible region of the spectrum, then the transmitted light has a colour complementary to the colour absorbed. Complementary colour is a colour generated from the wavelengths left after the absorption.
- 96. • The origin of colours of the coordination complexes can be very well explained by Crystal Field Theory. COLOURS IN COORDINATION COMPOUNDS: EXPLAINED BY CFT Eabs = Eeg - Et2g • When light is incident on the metal ion in octahedral complexes the electrons in the lower t2g orbitals absorb energy in the form of some wavelengths and get promoted to the higher eg sets of orbitals. The complementary colour of the absorbed colour becomes the colour of the complex. Thus the colour of the coordination compounds arises due to the transition of electrons between the split (n-1)d orbitals. Thus absorbed energy of light = • We know that in complexes, the five degenerate (n-1)d- orbitals split into two separate energy levels namely t2g and eg.
- 97. • It is important to note that in the absence of ligand, crystal field splitting does not occur and hence the substance is colourless. For example, removal of water from [Ti(H2O)6]Cl3 on heating renders it colourless. Similarly, anhydrous CuSO4 is white, but CuSO4.5H2O is blue in colour. Purple coloured Blue coloured Colourless Colourless
- 98. • [Ni(H2O)6]2+ complex is formed when NiCl2 is dissolved in water. It is green in colour. • If ethane-1,2-diamine(en) is progressively added in the molar ratios en:Ni = 1:1, 2:1, 3:1, the following series of complexes and their associated colour changes occur: THE INFLUENCE OF THE LIGAND ON THE COLOUR OF A COMPLEX No en added
- 99. COLOURS IN GEM STONES (1) Ruby is aluminium oxide (Al2O3) containing about 0.5-1% Cr3+ ions (d3), which are randomly distributed in positions normally occupied by Al3+ .We may view these Cr3+ ions as octahedral chromium(III) complexes incorporated into the alumina lattice; d–d transitions at these centres give rise to the colour. (2) In emerald Cr3+ ions occupy octahedral sites in the mineral beryl (Be3Al2Si6O18). The absorption bands here are yellow- red and blue, causing emerald to transmit light in the green region.
- 100. ADVANTAGES OF CFT The crystal field model is successful in explaining the following properties of coordination compounds: (1) Formation of coordination compounds (2) Structures of coordination compounds (3) colour of coordination compounds (4) magnetic properties of coordination compounds ADVANTAGES OF CFT
- 101. DRAWBACKS OF CFT There are mainly two drawbacks of CFT: 1. A negatively charged ligand should repel the d-electrons of metal more strongly as compared to neutral ligands. We know that greater the repulsion, larger will be the splitting of d- orbitals and such a ligand will be strong field ligand. But actually negatively charged ligands like F-, Cl- etc. are weak field ligands whereas neutral ligands like NH3, CO etc. are strong field ligands. This could not be explained by CFT. 2. In CFT, the metal-ligand bond is considered purely ionic but actually they exhibit covalent characters also. This also could not be explained by CFT.
- 102. BONDING IN METAL CARBONYLS The followings are some example of complexes with CO group as homoleptic ligand:
- 103. In a metal carbonyl, the metal-carbon bond possesses both σ and π character. The bond between the carbonyl molecule and the metal is further strengthened by the synergic effect (π back bonding) produced by the metal-ligand bond. These two types of bonding that exist in metal carbonyls are explained below: 2. When this happens there is very good overlap between the metal’s other d orbitals (dxy, dxz, dyz) with an empty pi* (antibonding) orbital on the carbonyl group. The overlap allows the metal to donate some of it’s excess electron density into the empty pi* (antibonding) carbonyl orbital. This forms a pi bond. The carbonyl is acting like a Lewis acid. This synergistic effect strengthens the M-CO interaction. It also weakens the CO triple bond (as electron density is being inserted into an antibonding orbital on CO). Structure of Metal Carbonyls: 1. A carbonyl group donates its lone pair of electrons to an empty metal d orbital. This is a sigma bond and involves the lone pair in the valence sigma orbital of the carbonyl and the dx2-y2 or dz2 (or sometimes p) metal orbitals. The carbonyl is acting like a Lewis base.
- 104. STABILITY OF COORDINATION COMPOUNDS The stability of a complex in solution refers to the degree of association between the two species involved in the state of equilibrium. The magnitude (stability or formation) of the equilibrium constant for the association, quantitatively expresses the stability. For a complex MLn , we can write: The instability constant or the dissociation constant of coordination compounds is defined as the reciprocal of the formation constant.
- 105. Free metal ions rarely exist in the solution so that M will usually be surrounded by solvent molecules which will compete with the ligand molecules, L, and be successively replaced by them. Thus we can write the formation of complex MLn in n steps and can write stability constant for each step as shown below: STEPWISE STABILITY CONSTANT
- 106. Since, water is in access, we can ignore the concentration of water and can write these reactions in terms of only ligand and metal as shown below:
- 107. Taking log of both the side we get the following expression: Example: We can calculate the overall stability constant of [Cu(NH3)4]2+ as follows:
- 109. APPLICATIONS OF COORDINATION COMPOUNDS For the year 2020-21, the applications in analytical Chemistry, metallurgy and biological systems are removed. So we will study only the applications in industry and in medicine.
- 110. APPLICATIONS OF COORDINATION COMPOUNDS IN INDUSTRY •Coordination compounds are used as catalysts for many industrial processes. Examples include rhodium complex, [(Ph3P)3RhCl], a Wilkinson catalyst, is used for the hydrogenation of alkenes. •Articles can be electroplated with silver and gold much more smoothly and evenly from solutions of the complexes, [Ag(CN)2]– and [Au(CN)2]– than from a solution of simple metal ions. •In black and white photography, the developed film is fixed by washing with hypo solution which dissolves the undecomposed AgBr to form a complex ion, [Ag(S2O3)2]3– .
- 111. APPLICATIONS OF COORDINATION COMPOUNDS IN MEDICINE •There is growing interest in the use of chelate therapy in medicinal chemistry. An example is the treatment of problems caused by the presence of metals in toxic proportions in plant/animal systems. Thus, excess of copper and iron are removed by the chelating ligands D–penicillamine and desferrioxime B via the formation of coordination compounds. EDTA is used in the treatment of lead poisoning. Some coordination compounds of platinum effectively inhibit the growth of tumours. Examples are: cis–platin and related compounds.