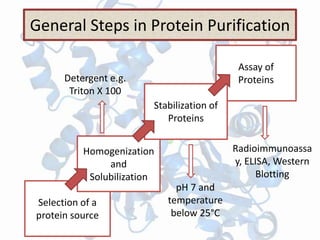
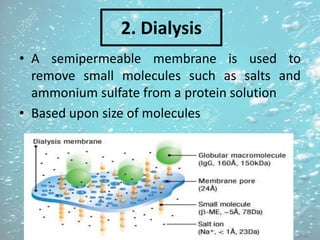
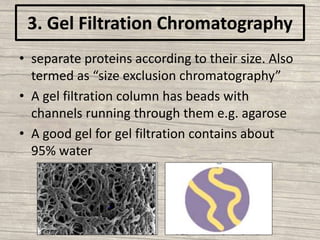
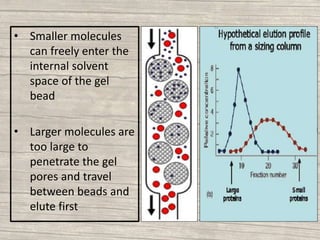
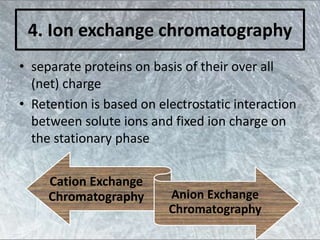
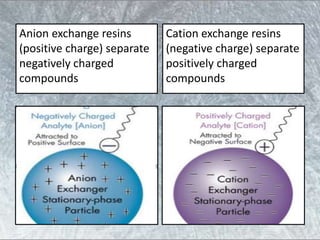
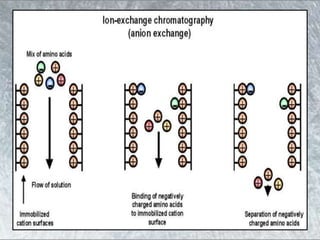
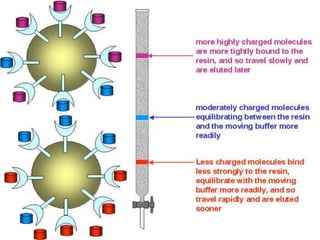
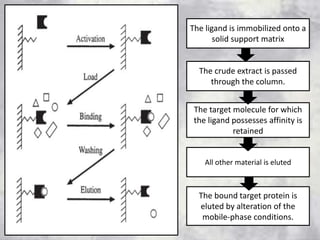
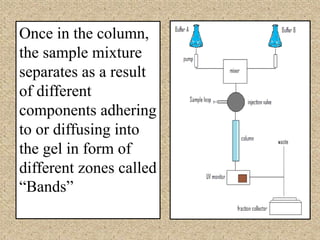
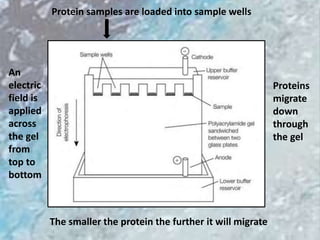
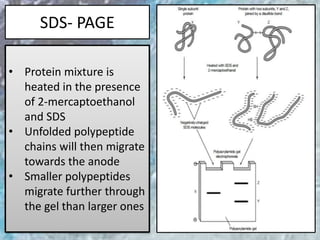
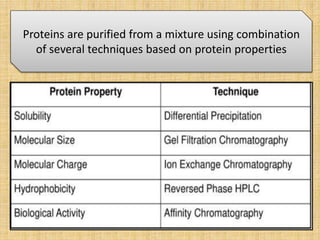
The document discusses various techniques for purifying proteins. General steps in protein purification include selecting a protein source, homogenizing and solubilizing the proteins, and stabilizing the proteins. Specific techniques discussed include ammonium sulfate precipitation, dialysis, gel filtration chromatography, ion exchange chromatography, affinity chromatography, fast performance liquid chromatography, and gel electrophoresis. The goal of protein purification is to isolate a single protein of interest from other contaminating proteins in order to study its structure and function.