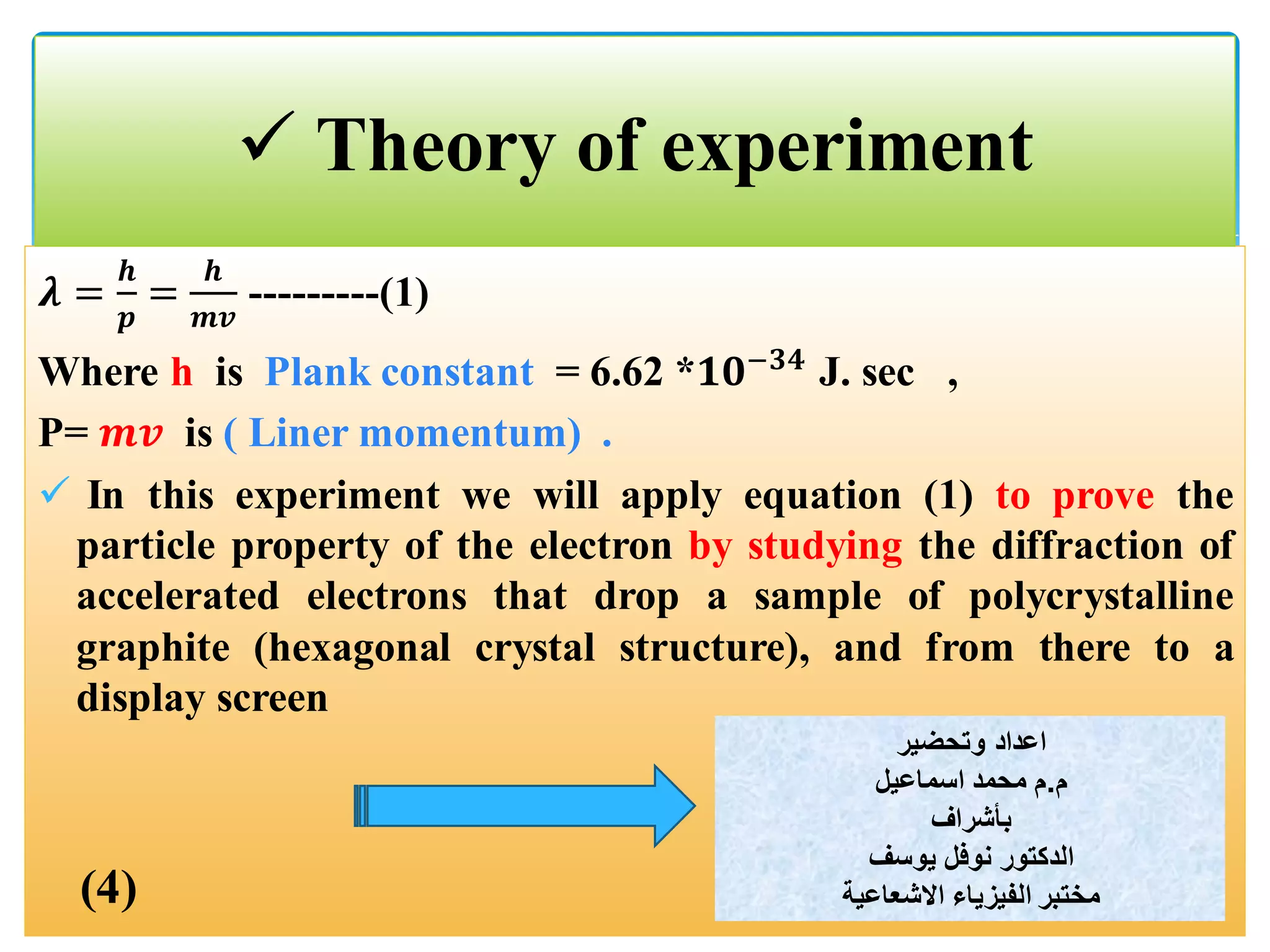
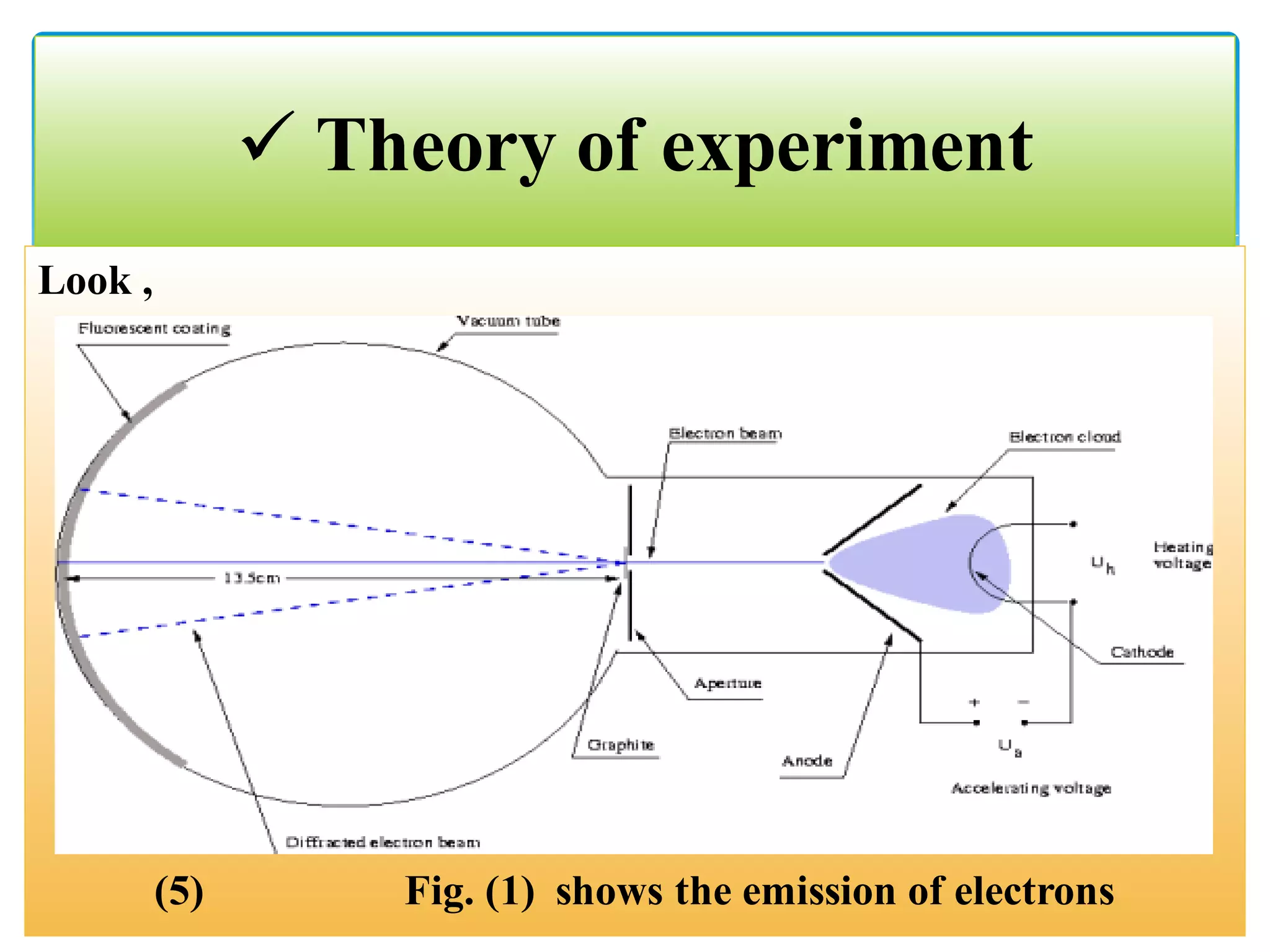
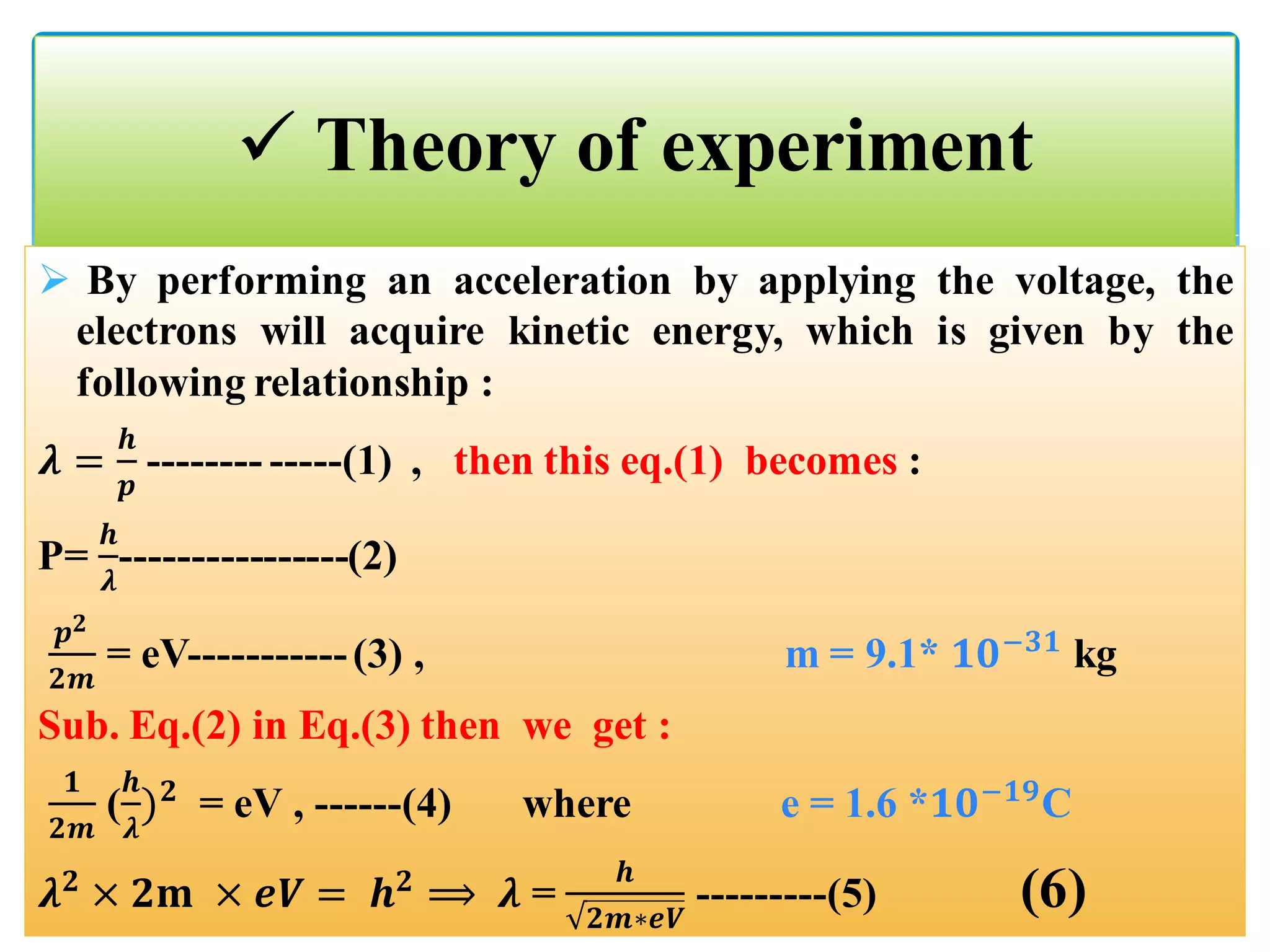
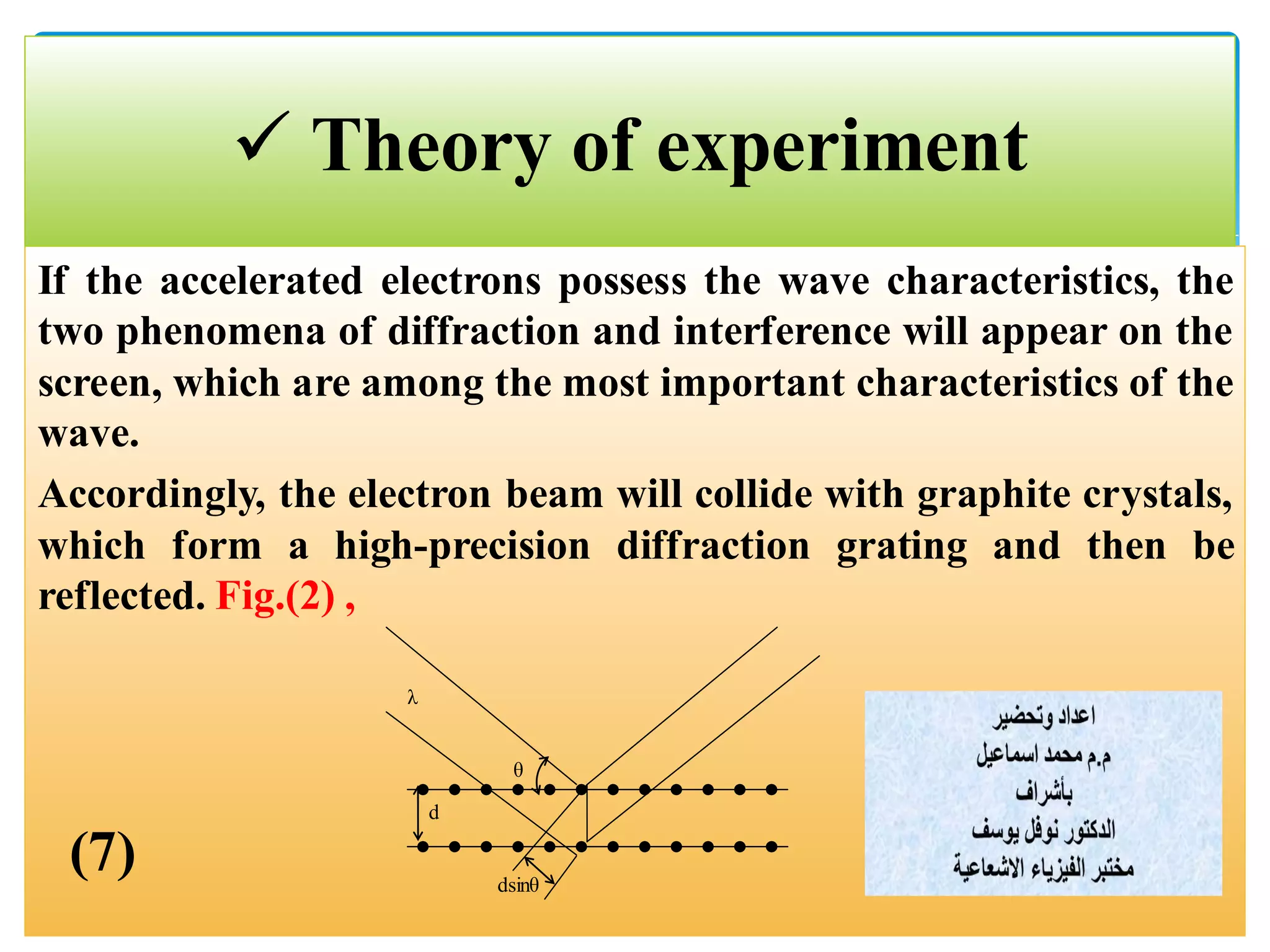
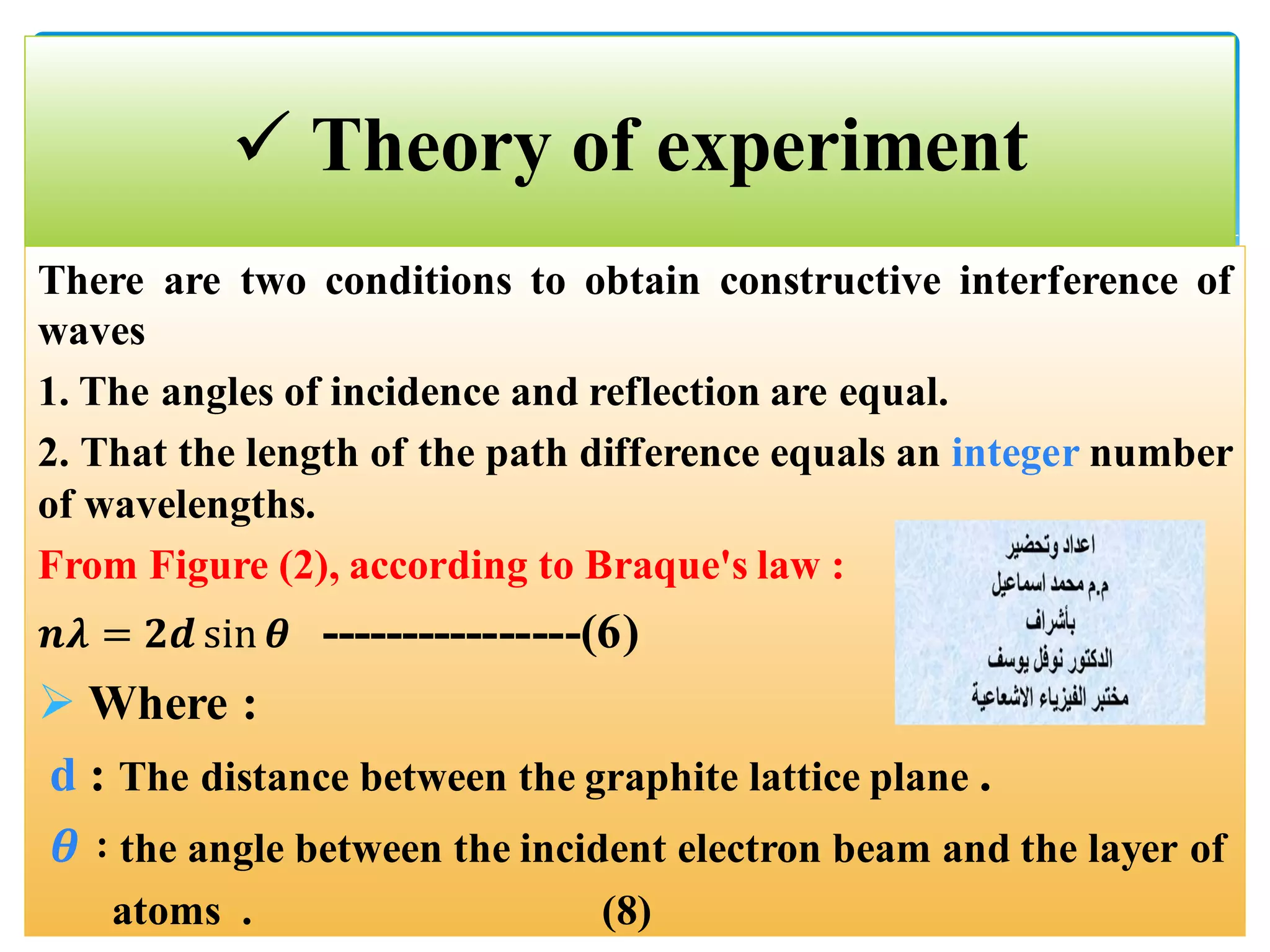
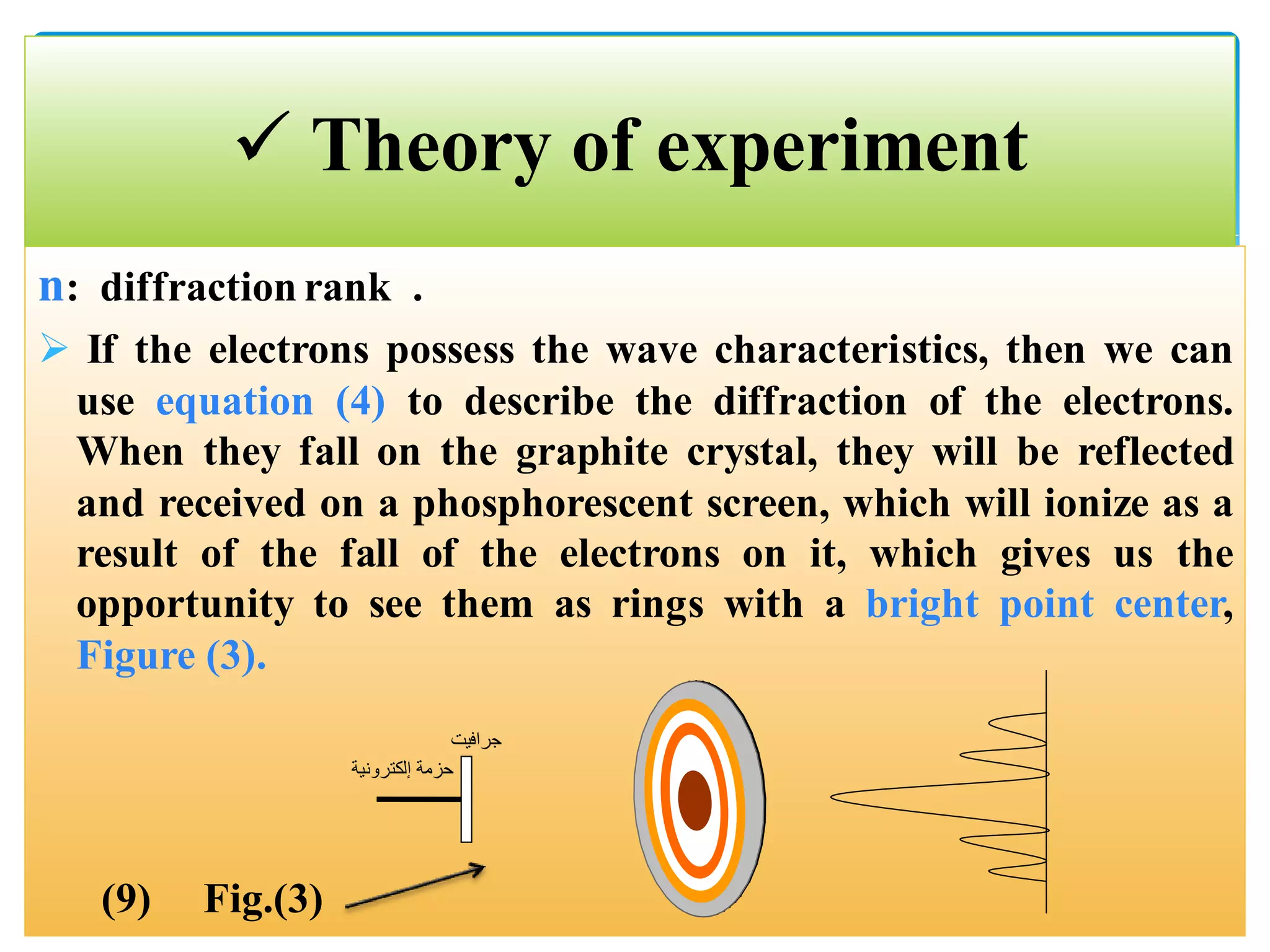
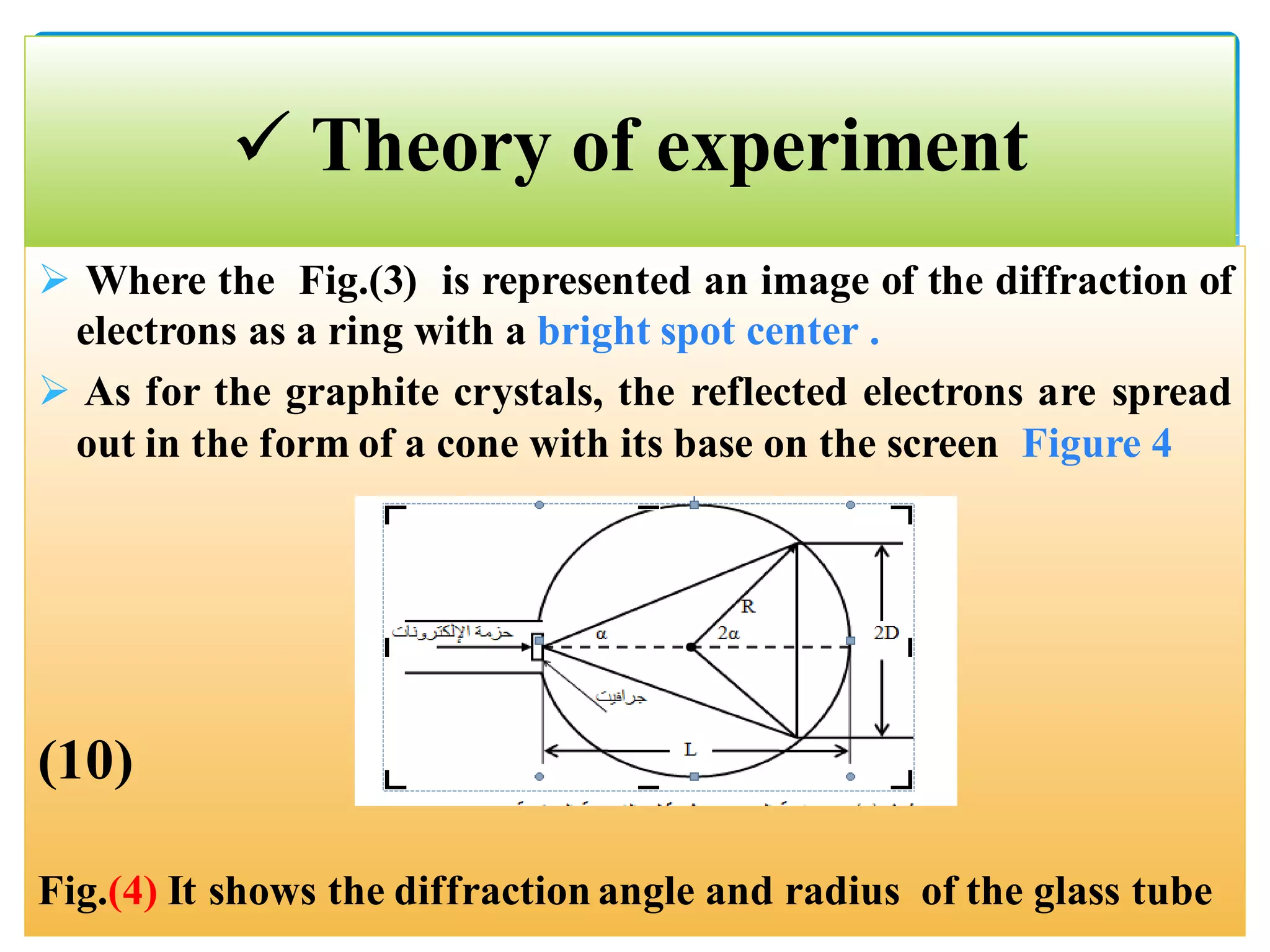
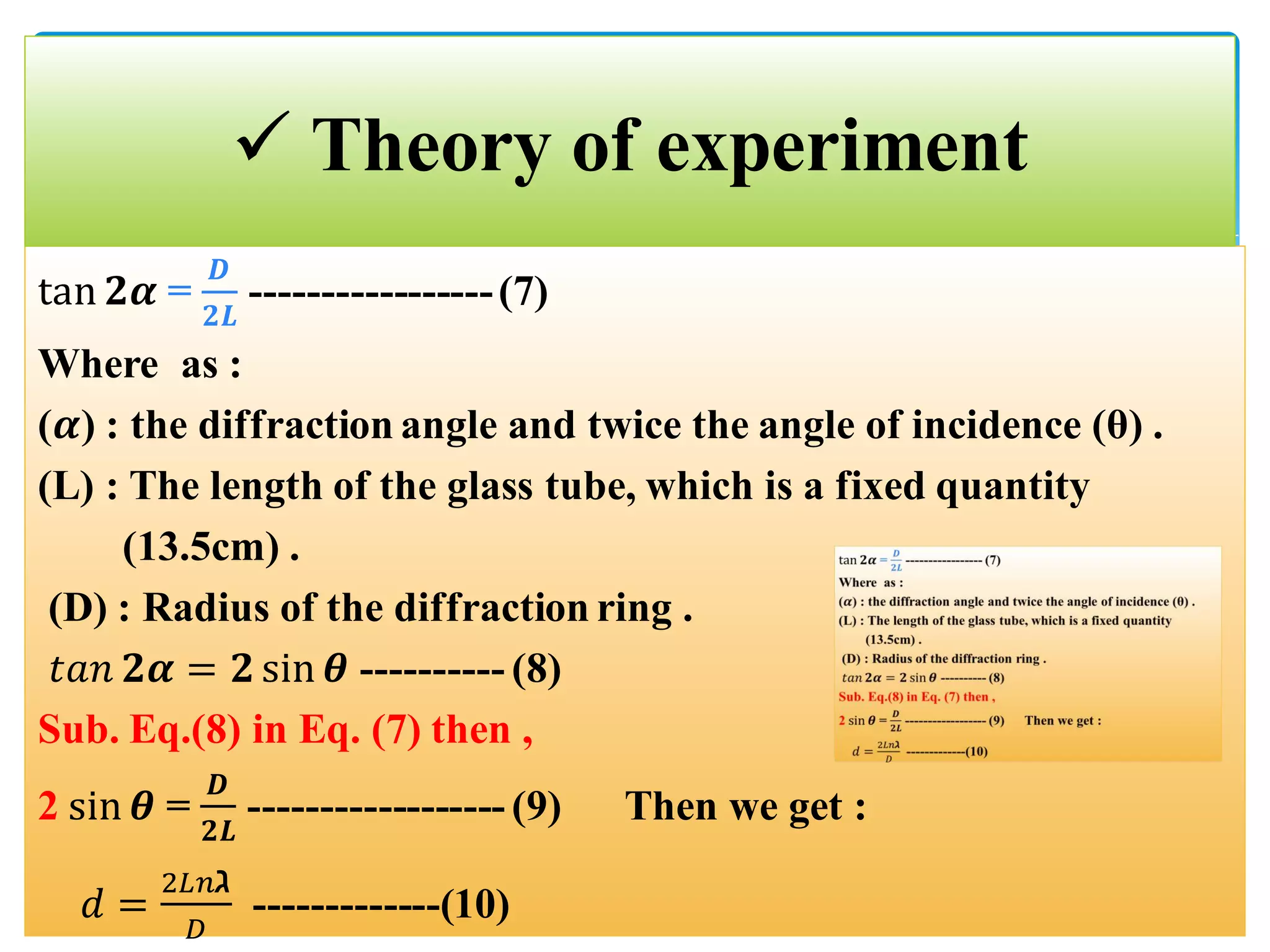
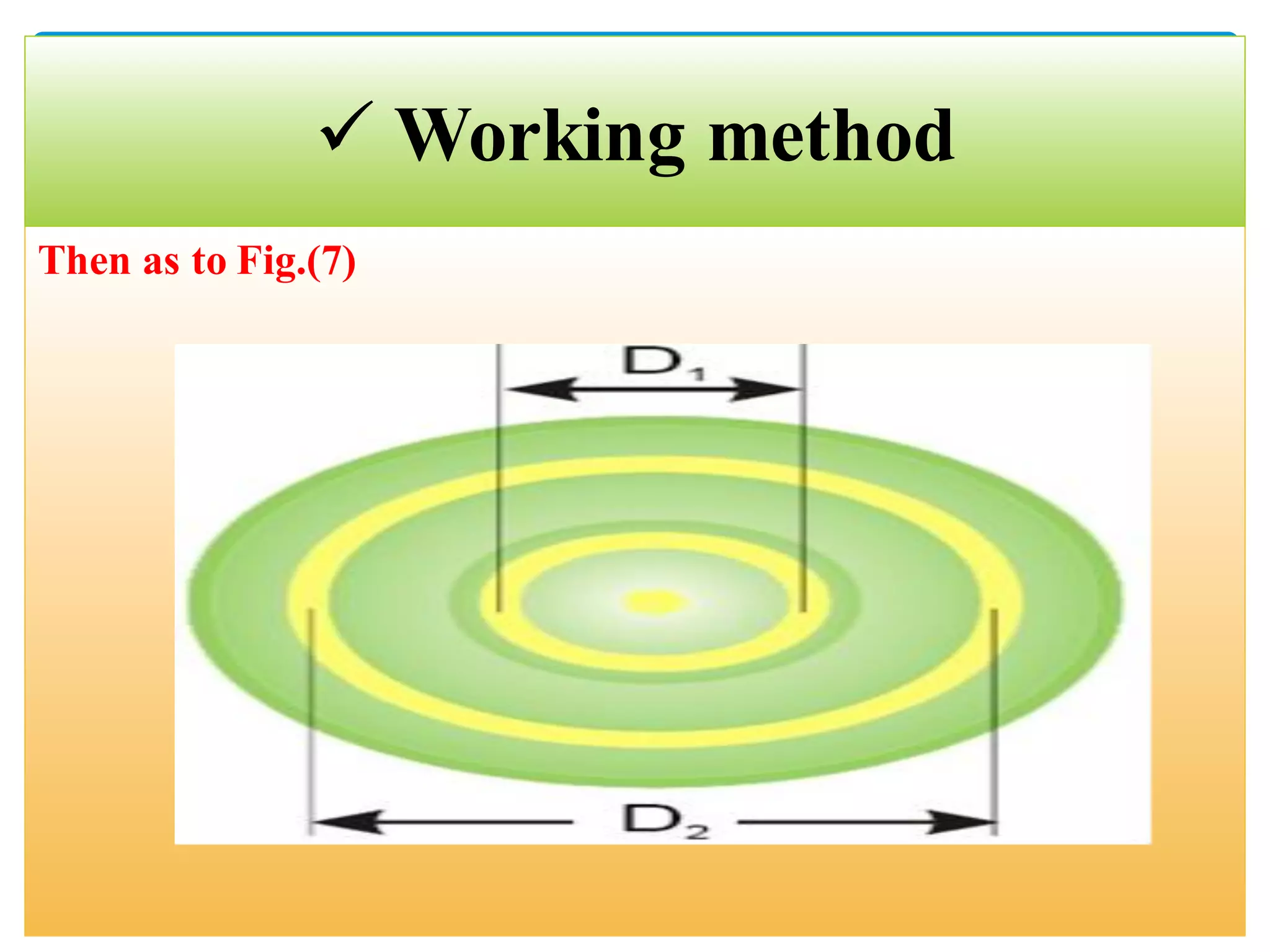
The document describes an experiment to demonstrate the wave-particle duality of electrons. Electrons are fired at a graphite crystal, causing diffraction that can be observed on a screen. By varying the acceleration voltage and measuring the diameters of diffraction rings, the wavelength of the electrons is calculated using Bragg's law. A graph of diameter versus wavelength shows a direct relationship, supporting the concept of electrons behaving as waves. The experiment thus achieves the aims of finding the electron wavelength and measuring interlayer spacing in the graphite crystal.