Embed presentation
Downloaded 1,653 times

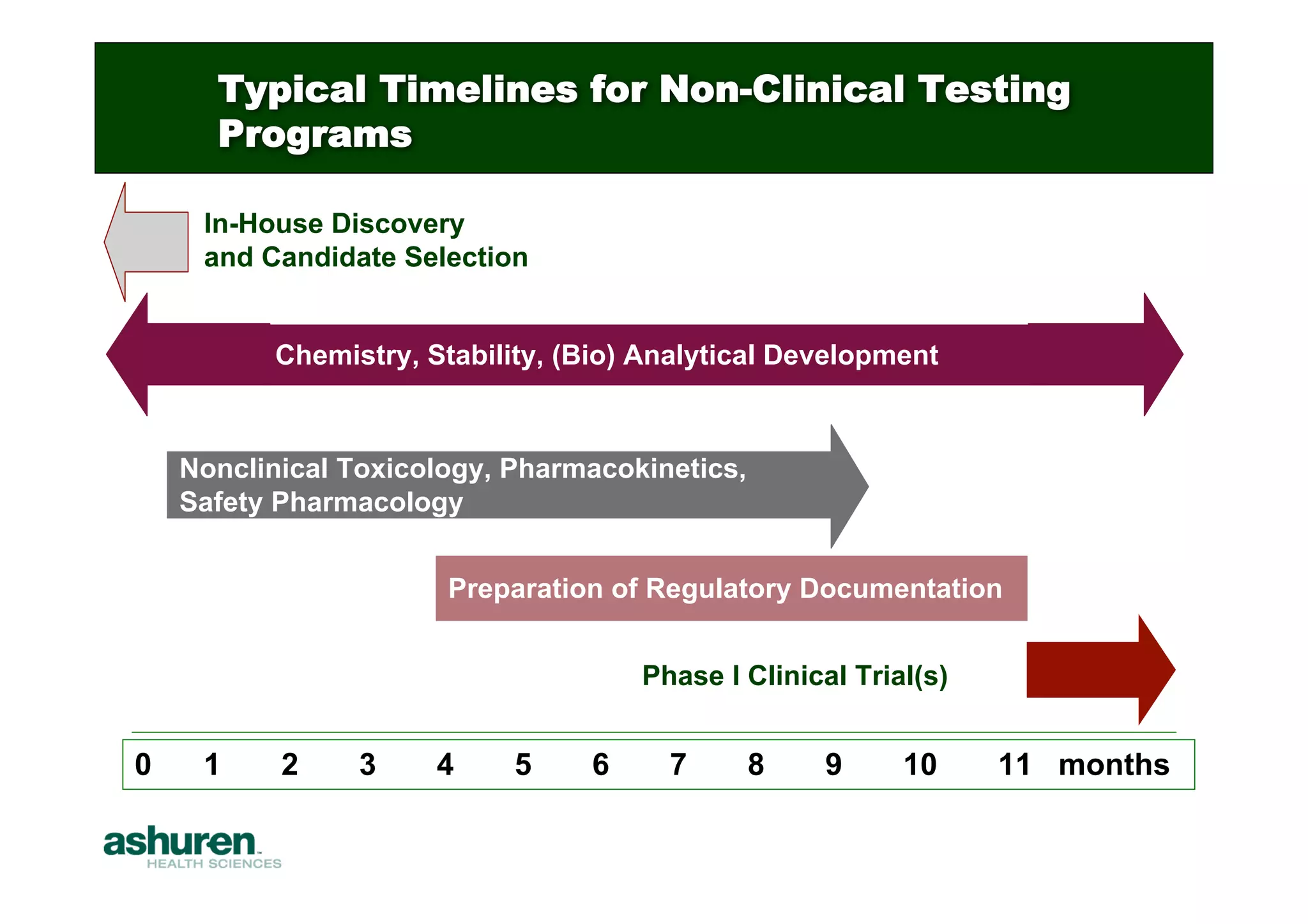







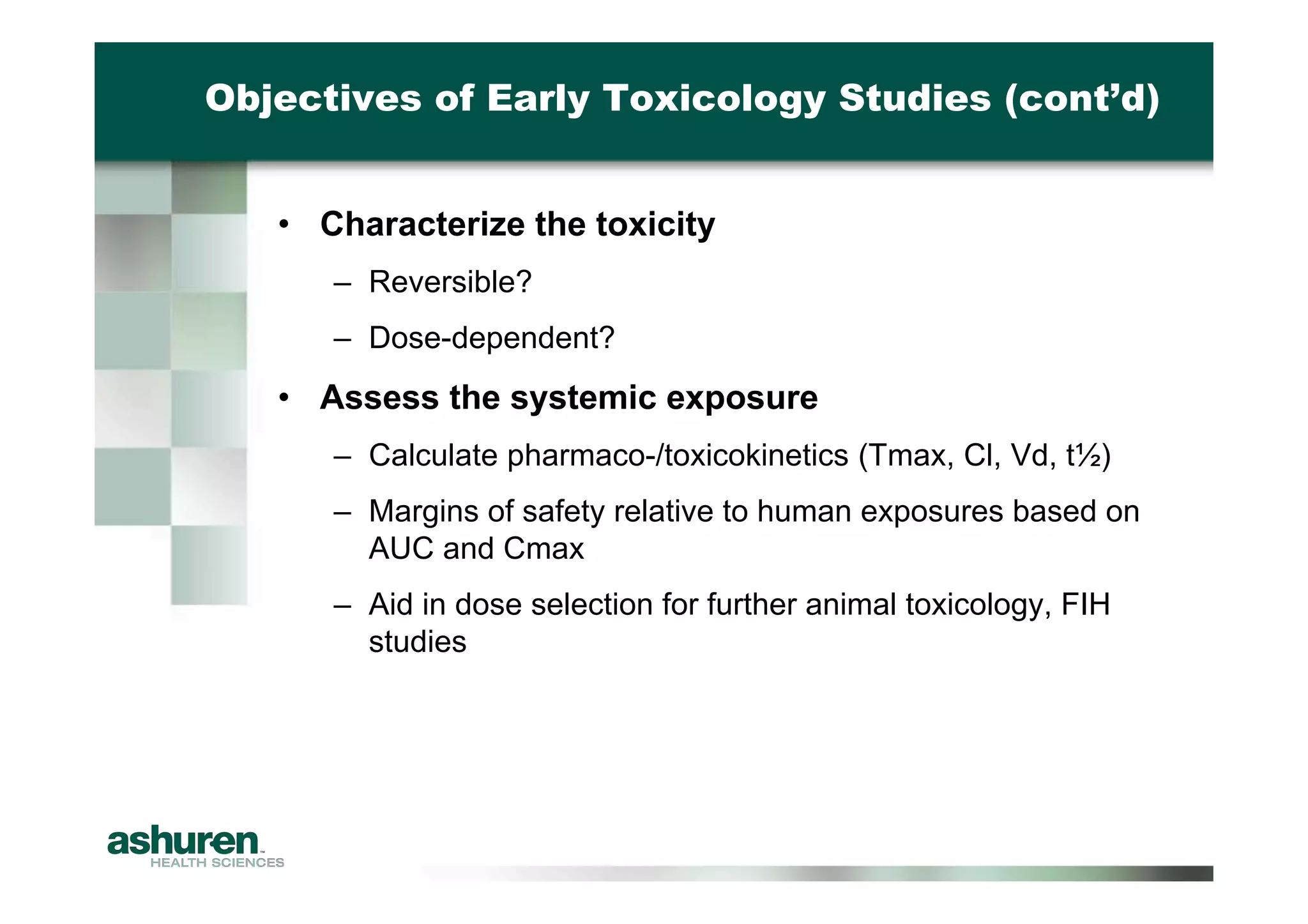
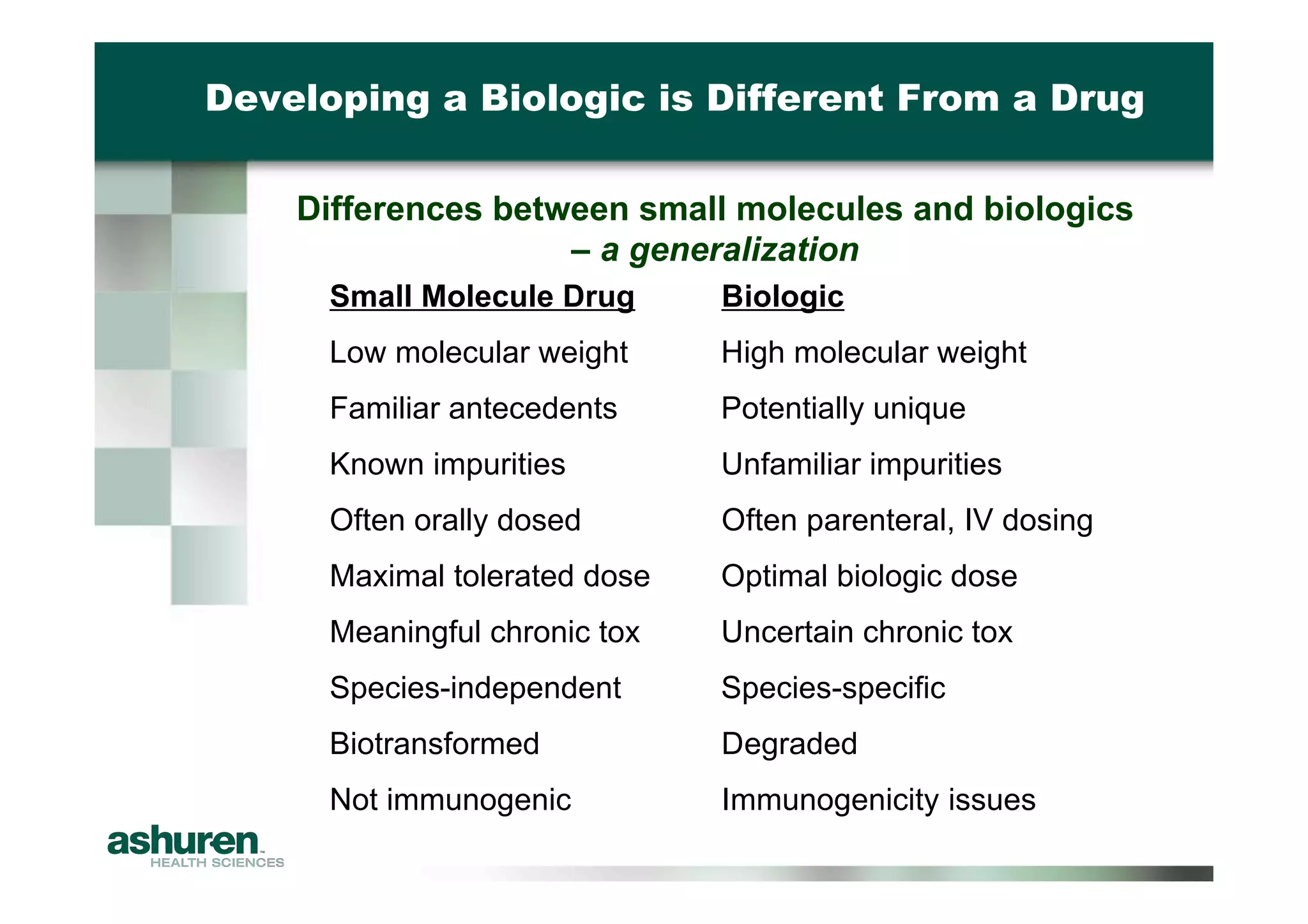
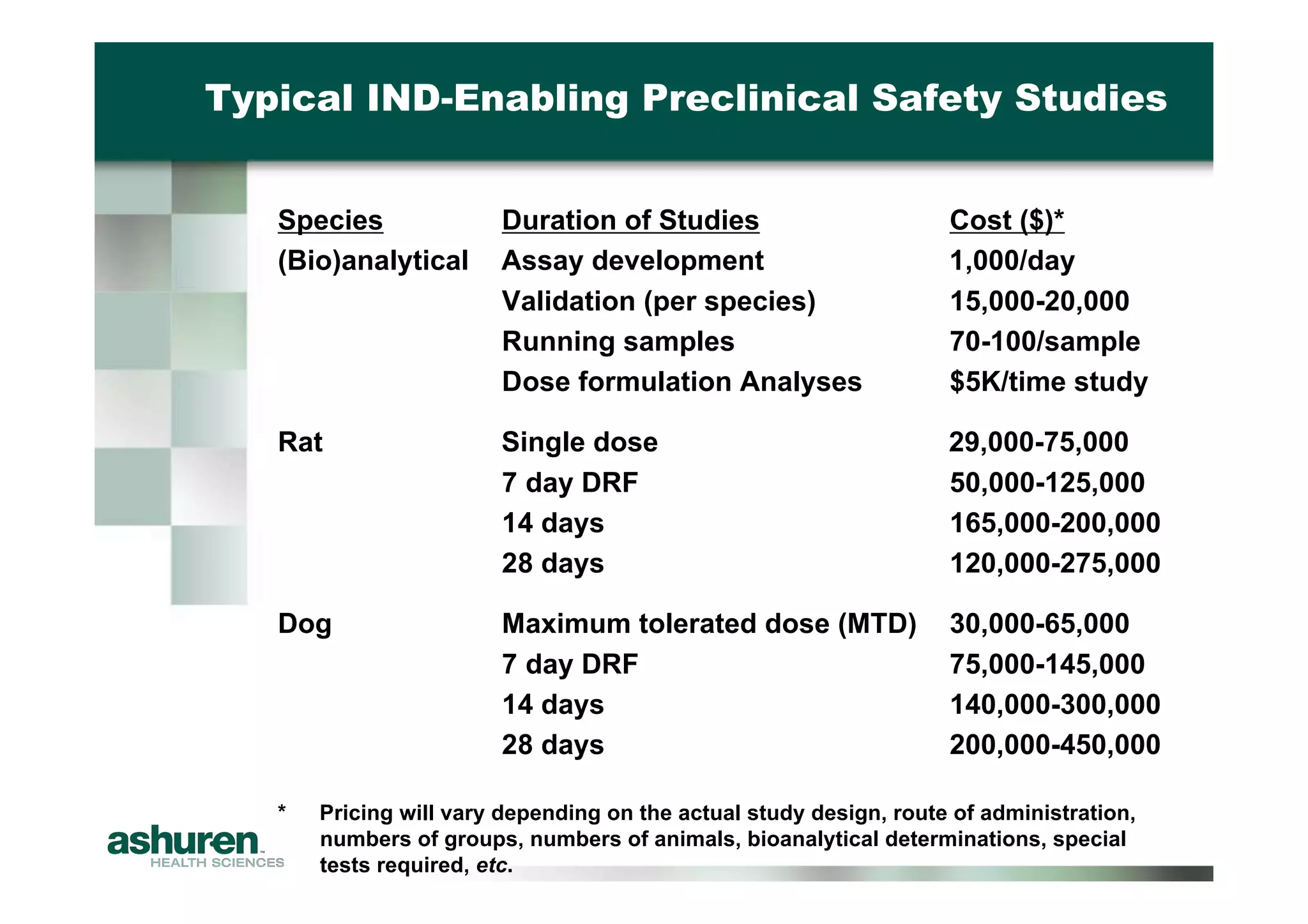
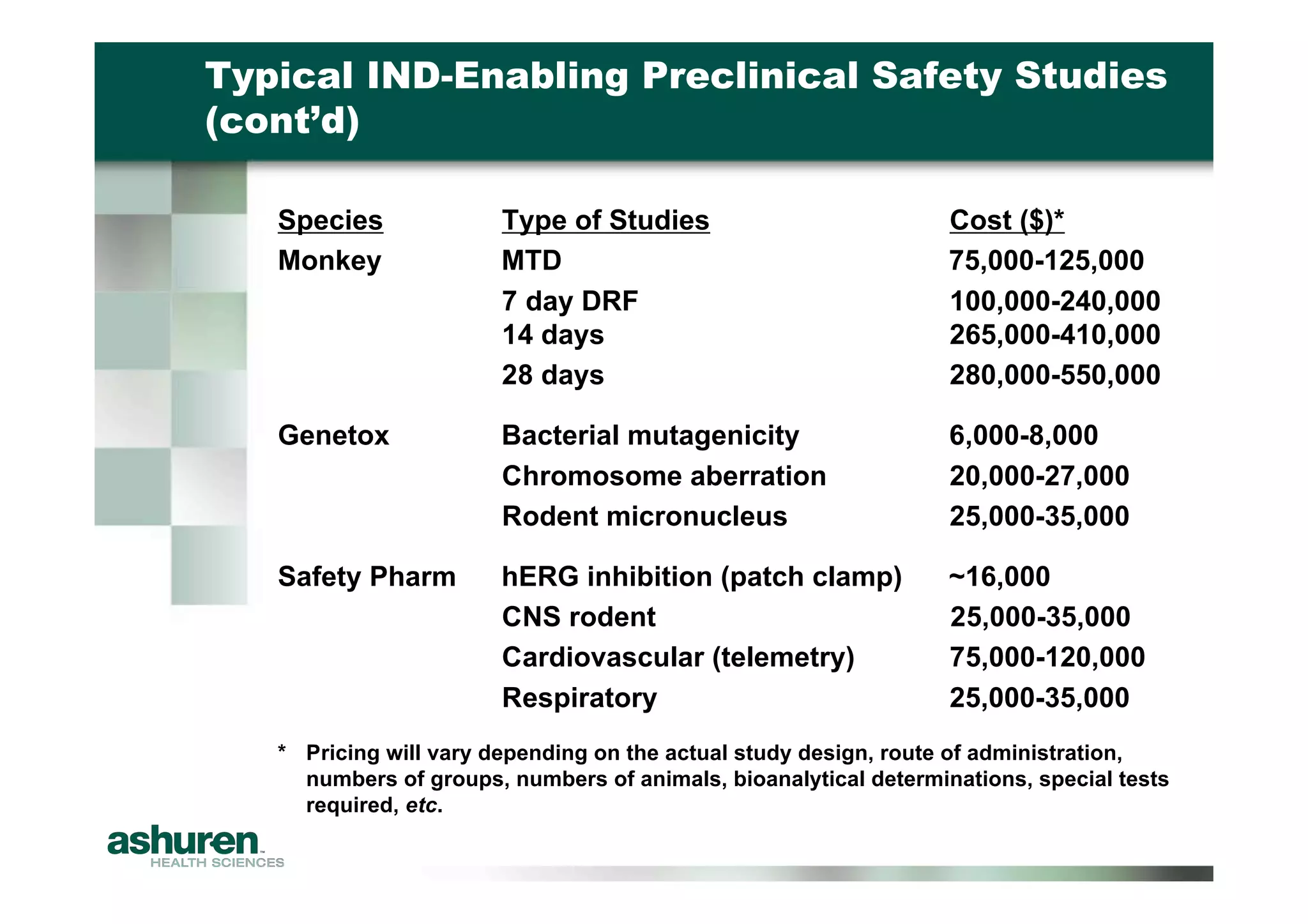
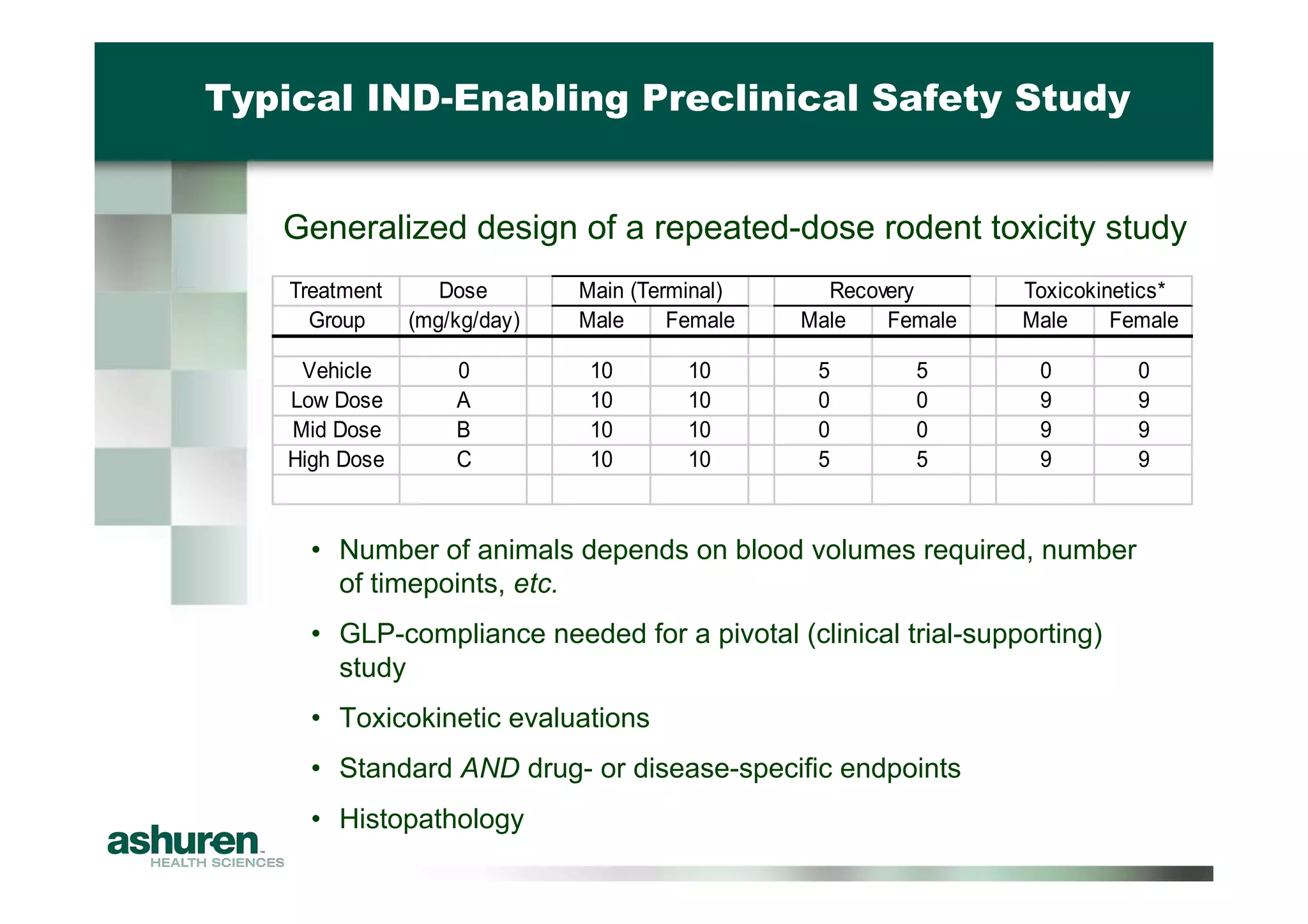
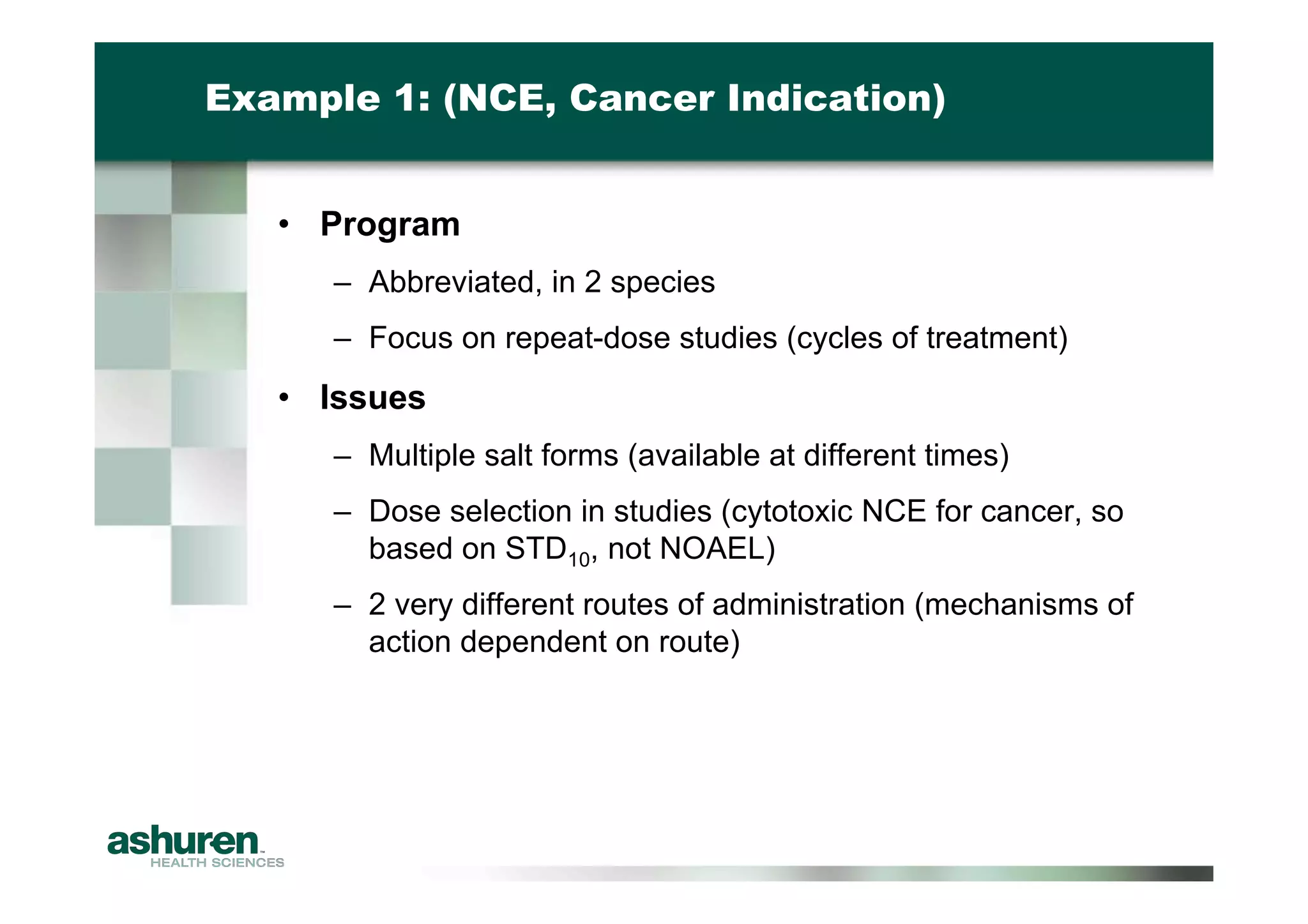
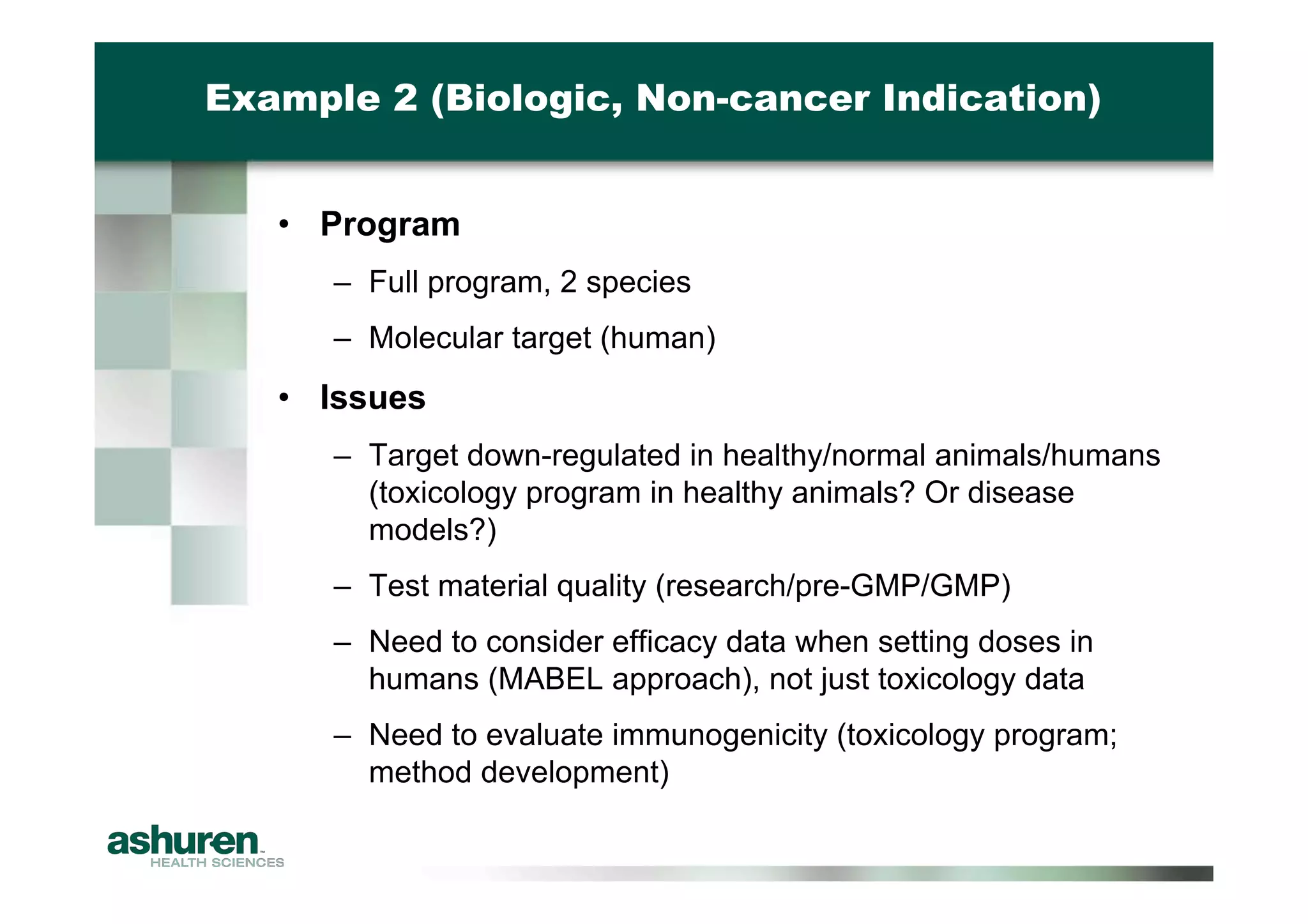






This document outlines the essential considerations for planning preclinical development programs for emerging pharmaceutical and biotech firms aiming for first-in-human studies. It highlights the need for a well-structured approach to nonclinical testing, regulatory documentation, and toxicology studies, emphasizing the importance of efficient resource management and expert interpretation of results. Successful navigation of preclinical development is portrayed as a high-cost investment requiring careful planning to meet regulatory expectations and achieve clinical trial readiness.
























