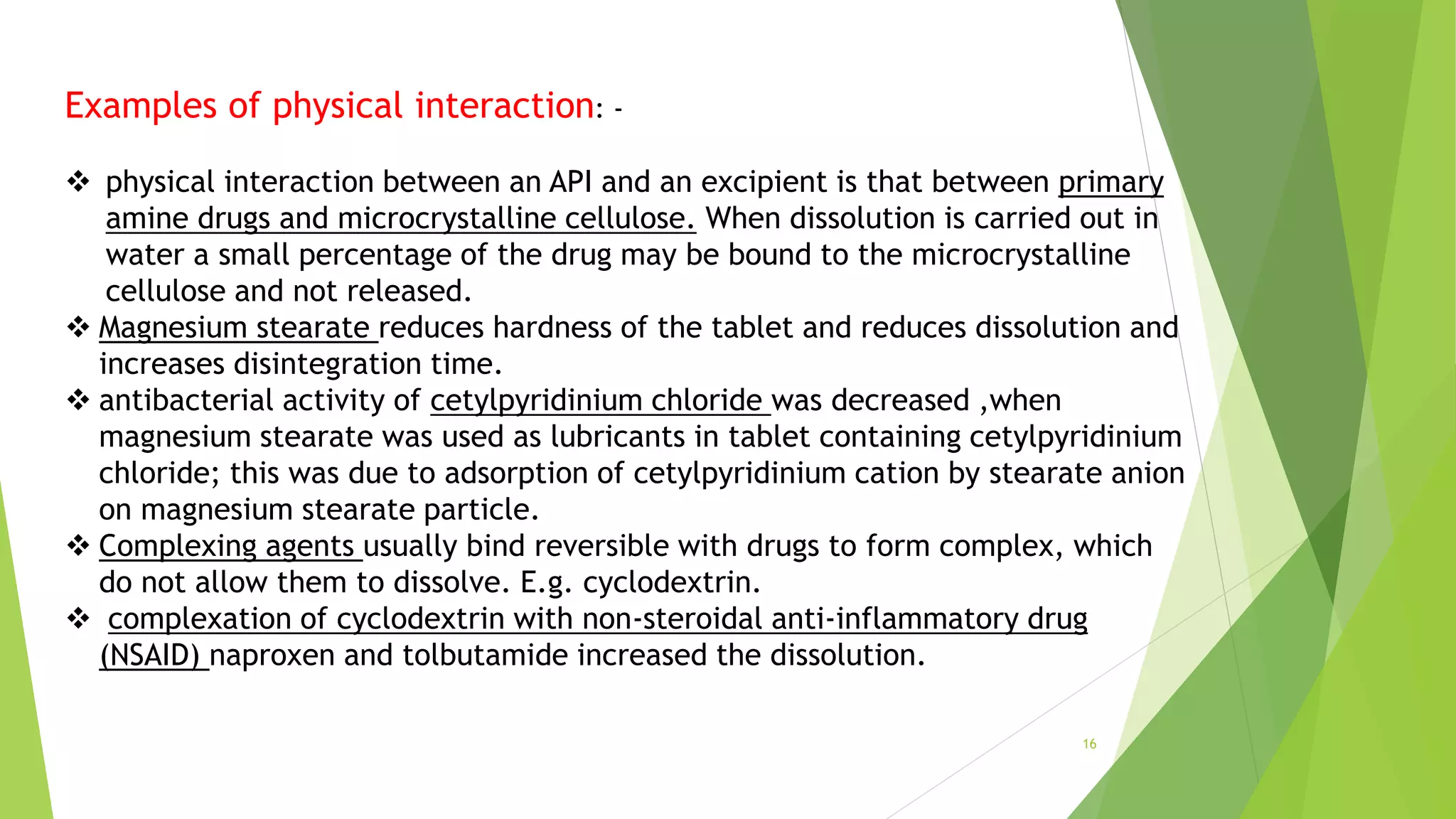
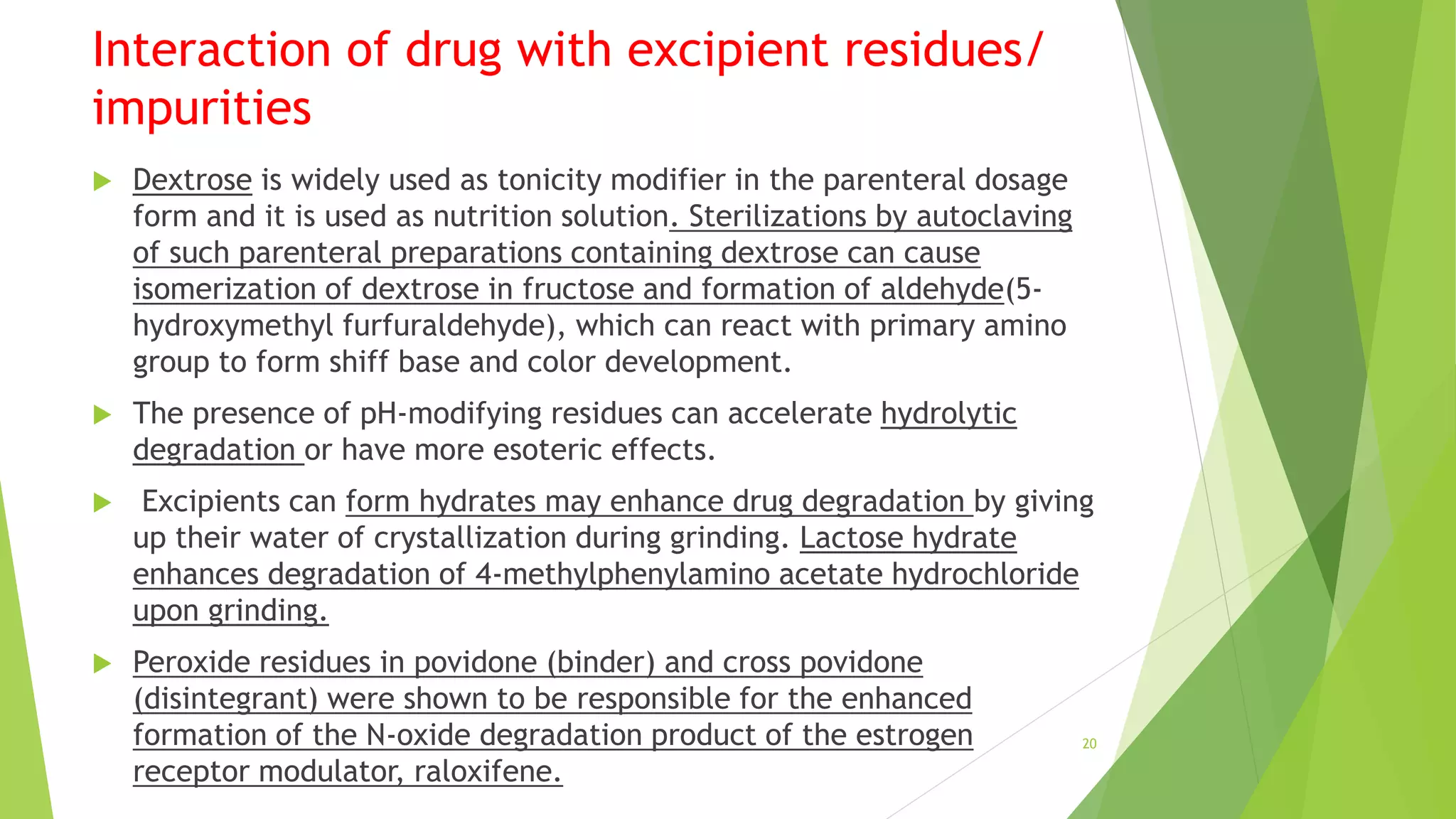
This document discusses drug-excipient interactions that can occur in pharmaceutical formulations. It defines excipients as substances other than the active pharmaceutical ingredient that are included in drug products. Excipients can interact physically or chemically with drugs in ways that are either beneficial or detrimental to the drug's effectiveness. Physical interactions do not involve chemical changes but can impact properties like drug dissolution. Chemical interactions result in chemical reactions between the drug and excipient or impurities that can produce degradation products. The document provides examples of specific physical and chemical interactions and mechanisms like hydrolysis, oxidation, and isomerization through which interactions can occur.