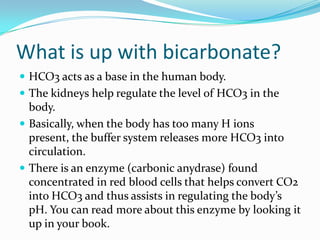
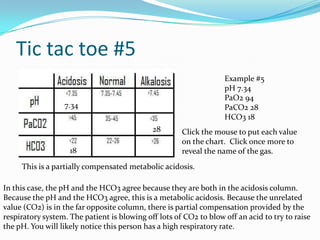
This document provides an overview of ABG (arterial blood gas) interpretation. It begins by explaining the importance of maintaining pH homeostasis and the roles of carbon dioxide (CO2) and bicarbonate (HCO3) in buffering pH. It then discusses acid-base imbalances including respiratory and metabolic acidosis and alkalosis. The document uses a "tic-tac-toe" chart to demonstrate how to determine if an ABG result is normal, acidotic, or alkalotic; respiratory or metabolic; and uncompensated, partially compensated, or fully compensated based on the pH, PaCO2, PaO2, and HCO3 levels. Examples are provided to illustrate each