(250ml/5mins)
250ml/ 5mins or transfusion
1) This document provides guidance on evaluating and treating shock in patients. It discusses the different types of shock (hypovolemic, cardiogenic, distributive), their causes, signs, and treatments.
2) The initial approach involves taking a thorough history, performing a full physical exam, and obtaining basic lab tests to help identify the type and cause of shock.
3) Mixed venous oxygen saturation (SvO2) and central venous pressure (CVP) measurements can help guide fluid resuscitation and vasopressor use depending on whether the values are low or normal. Fluid challenges are recommended initially














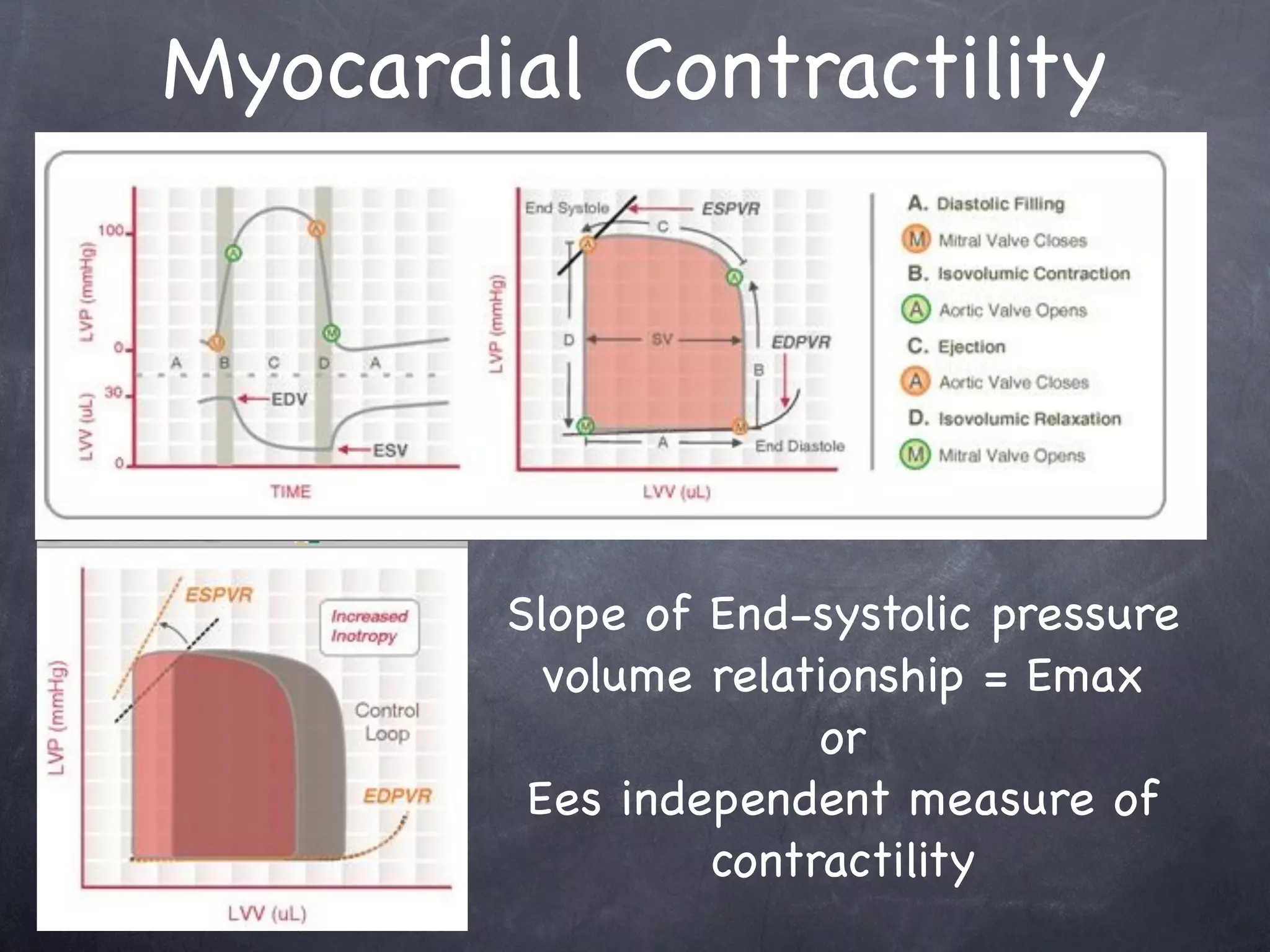
![Physiologic Determinants
Systemic Vascular Resistance (SVR)
Vessel length - or length of vessel bed
Blood viscosity - incr Hgb => incr resistance
Vessel diameter - majority of resistance in
small arteriolar vessels (resistance vessels)
- 450 to 100 µm
SG definition = 80*[(MAP-MPAOP)/CO]](https://image.slidesharecdn.com/shock2011-13262570787807-phpapp01-120110224547-phpapp01/75/Shock-2011-15-2048.jpg)



![Newer Non-Invasive
Hemodynamic Measures
Systolic Pressure Variation (MaxSBP-MinSBP)
Pulse Pressure Variation[(MaxArterialPulsePressure-
MinArterialPulsePressure)/MeanArterialPulsePressure]
Stroke Volume Variation [(MaxSV-MinSV)/MeanSV]
Measurements made during one controlled vent breath
If SPV>10, SVV>10%, PPV>13% => Volume Responsiveness](https://image.slidesharecdn.com/shock2011-13262570787807-phpapp01-120110224547-phpapp01/75/Shock-2011-20-2048.jpg)





















































































































































