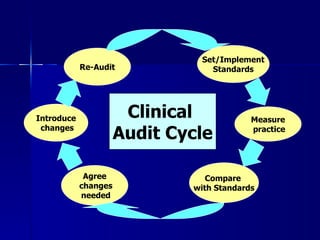
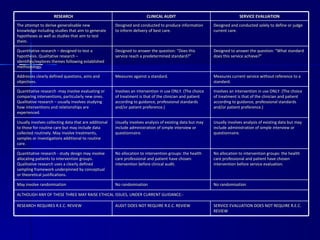
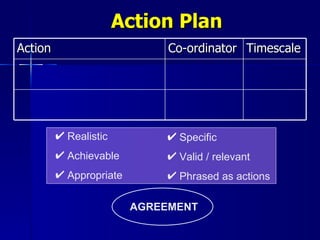
Clinical audit is a quality improvement process aimed at enhancing patient care by systematically evaluating healthcare practices against established criteria. It differs from research as it focuses on measuring compliance with best practices rather than discovering new knowledge. The process involves selecting areas of care for review, implementing changes, and continuously monitoring for improvements in healthcare delivery.