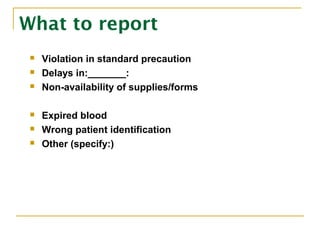
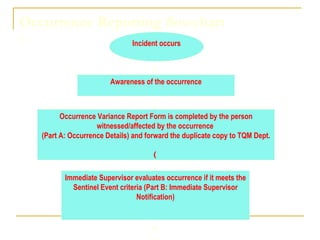
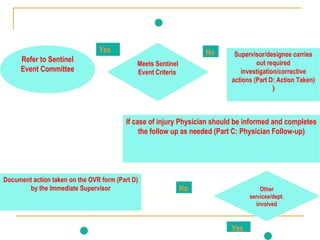
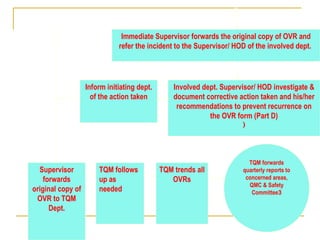
The document outlines the policies and procedures for reporting occurrences and sentinel events at a hospital using Occurrence Variance Reports (OVR). It states that OVRs should be completed by staff to document any incidents, injuries, or issues. Sentinel events involving major patient harm or death require special reporting to the Quality Improvement Coordinator and Sentinel Event Committee for a root cause analysis and action plan. The Total Quality Management Department monitors OVRs, identifies trends, and reports to relevant committees to prevent future issues. All occurrence reporting and investigation information is kept confidential by the TQM department.