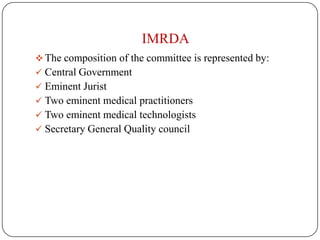
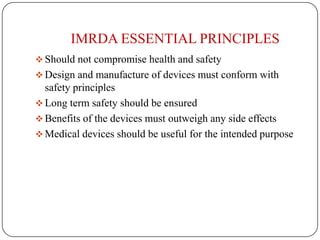
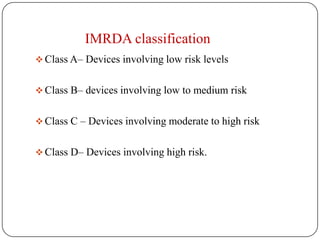
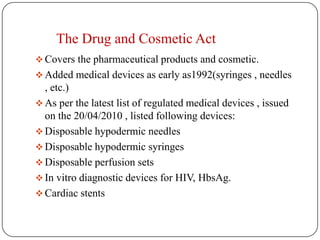
The document discusses medical device regulation in India. It provides definitions of medical devices and outlines the regulatory bodies that govern them, including the Central Drugs Standard Control Organization (CDSCO). It describes the proposed Indian Medical Devices Regulatory Act (IMRDA) and its objectives to establish standards, classify devices by risk, and regulate safety. The regulation of medical devices in India is still developing, with proposals to expand regulation beyond the limited devices currently covered under the Drugs and Cosmetics Act.