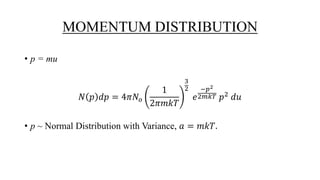
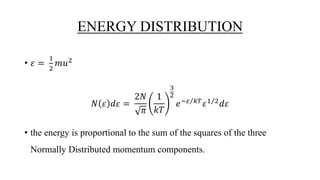
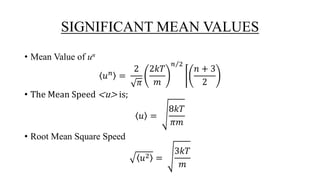
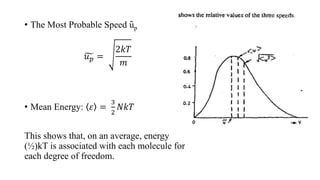
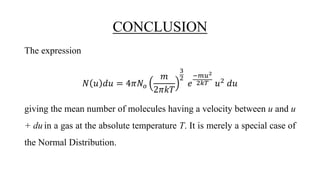
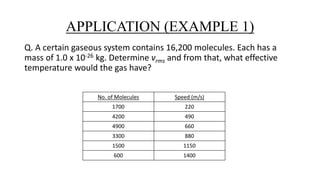
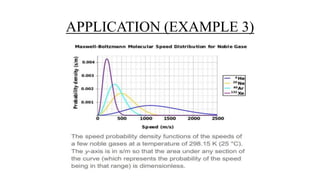
The document discusses the Maxwell-Boltzmann velocity distribution, covering its introduction, derivation, and application examples. It explores kinetic theory, probability assumptions, and provides mathematical expressions for momentum and energy distributions in gases. Applications include calculating the root mean square speed and effective temperature of gas particles based on their velocities.