Embed presentation
Downloaded 16 times

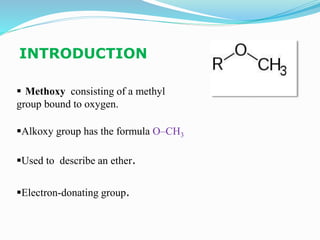
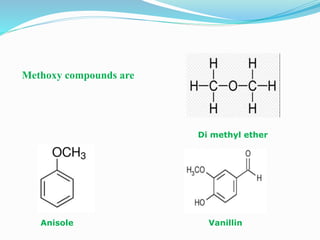

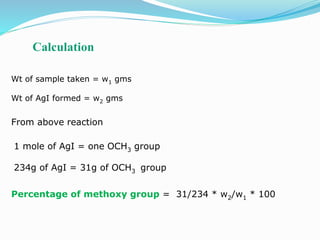
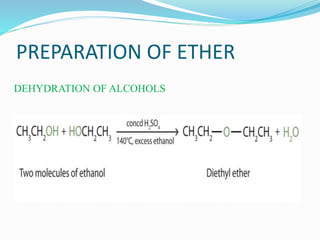
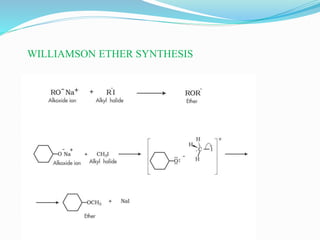
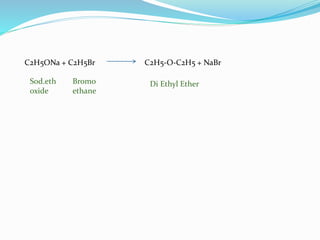

The document discusses methoxy groups, which consist of a methyl group bound to oxygen. It provides examples of methoxy compounds like dimethyl ether and anisole. It then describes Zeisel's method for estimating the number of methoxy groups in a compound, which involves reaction with hydroiodic acid to liberate the methoxy group as methyl iodide, which precipitates as silver iodide upon reaction with silver nitrate. The amount of silver iodide precipitate corresponds to the number of methoxy groups present. It also briefly discusses the preparation of ethers through dehydration of alcohols and the Williamson ether synthesis reaction.








