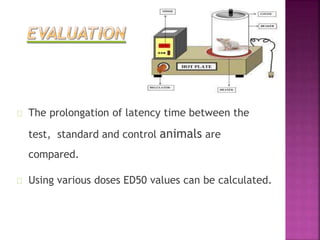
This document discusses various preclinical screening methods for analgesic drugs. It describes several in vivo animal models used to test analgesics, including thermal, electrical, chemical and mechanical stimulus-induced pain models. The hot plate, tail flick and tail immersion tests involve thermal stimuli. The tooth pulp stimulation and tail shock tests use electrical stimuli. The formalin and writhing tests employ chemical stimuli. Mechanical stimuli models include the tail clip and Randall Selitto tests. The models aim to evaluate potential analgesic drugs for their ability to prolong response latency and reduce pain-related behaviors like paw licking.