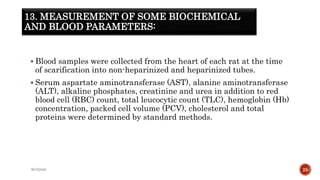
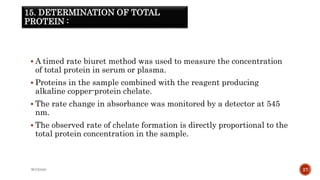
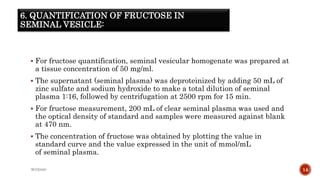
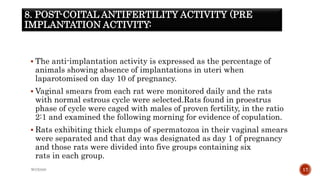
The document discusses in vivo evaluation techniques for antifertility agents using female and male rats. It outlines various methods for assessing antifertility activity, including hormonal analysis, mating trials, and body weight measurements, as well as specific procedures for drug administration and observation. It also includes references for further reading on medical pharmacology and pharmacognosy.



















![ The ovaries weight variations prior to and after treatment with extracts were
calculated.
Percentage inhibition of ovarian weight was calculated using the following equation:
Percentage inhibition in ovarian weight = [1 - (XE - C)]/E- Cx 100.
Where, C = mean ovarian weight from rats treated with vehicle,
E = estradiol and-
XE = the mean ovarian weight of rats treated with
extract and estradiol
WOX888 22](https://image.slidesharecdn.com/antifertilityscreening1-231212184137-38697e1f/85/In-vivo-evaluation-techniques-for-Antifertility-agent-activity-22-320.jpg)