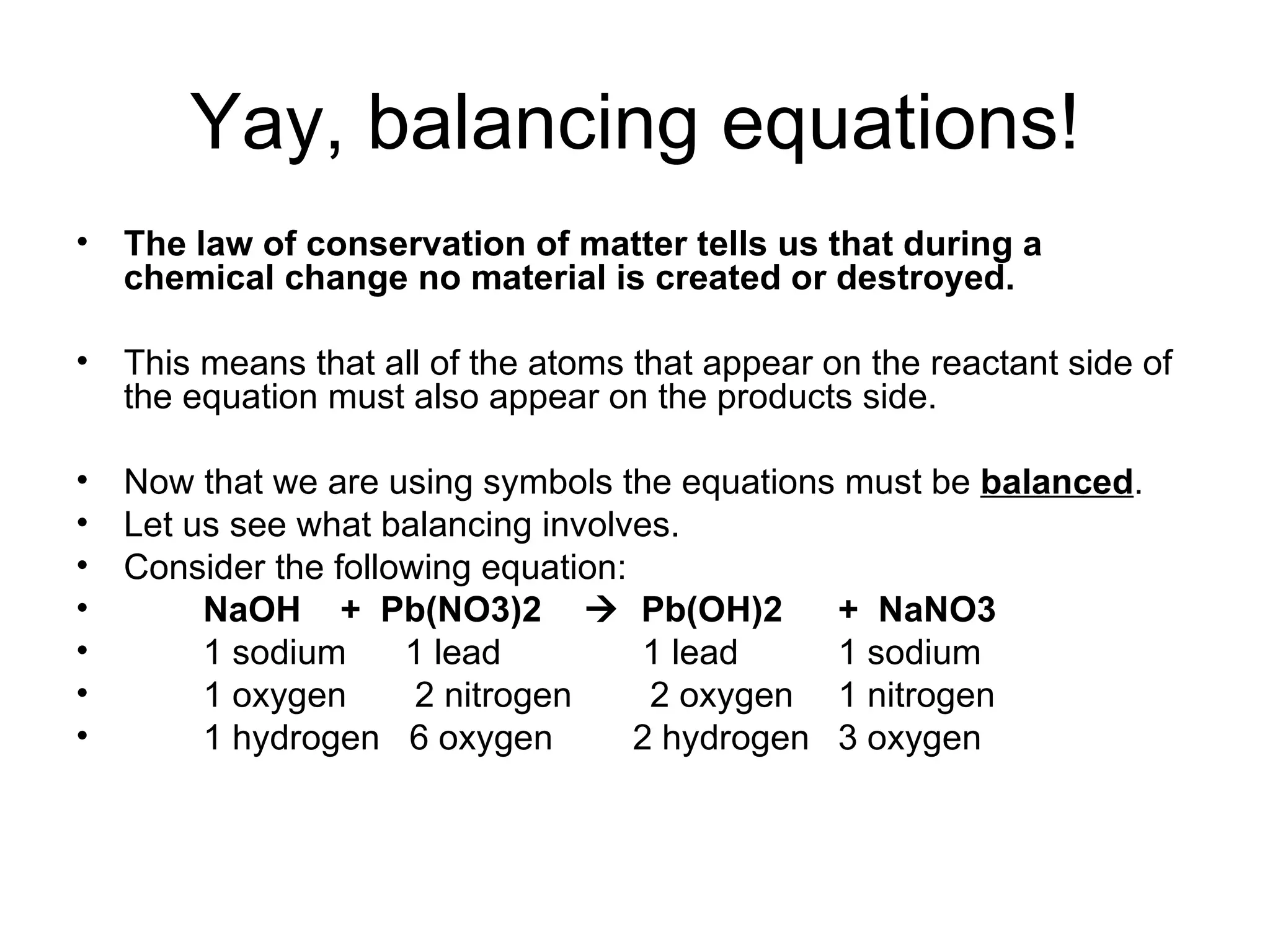
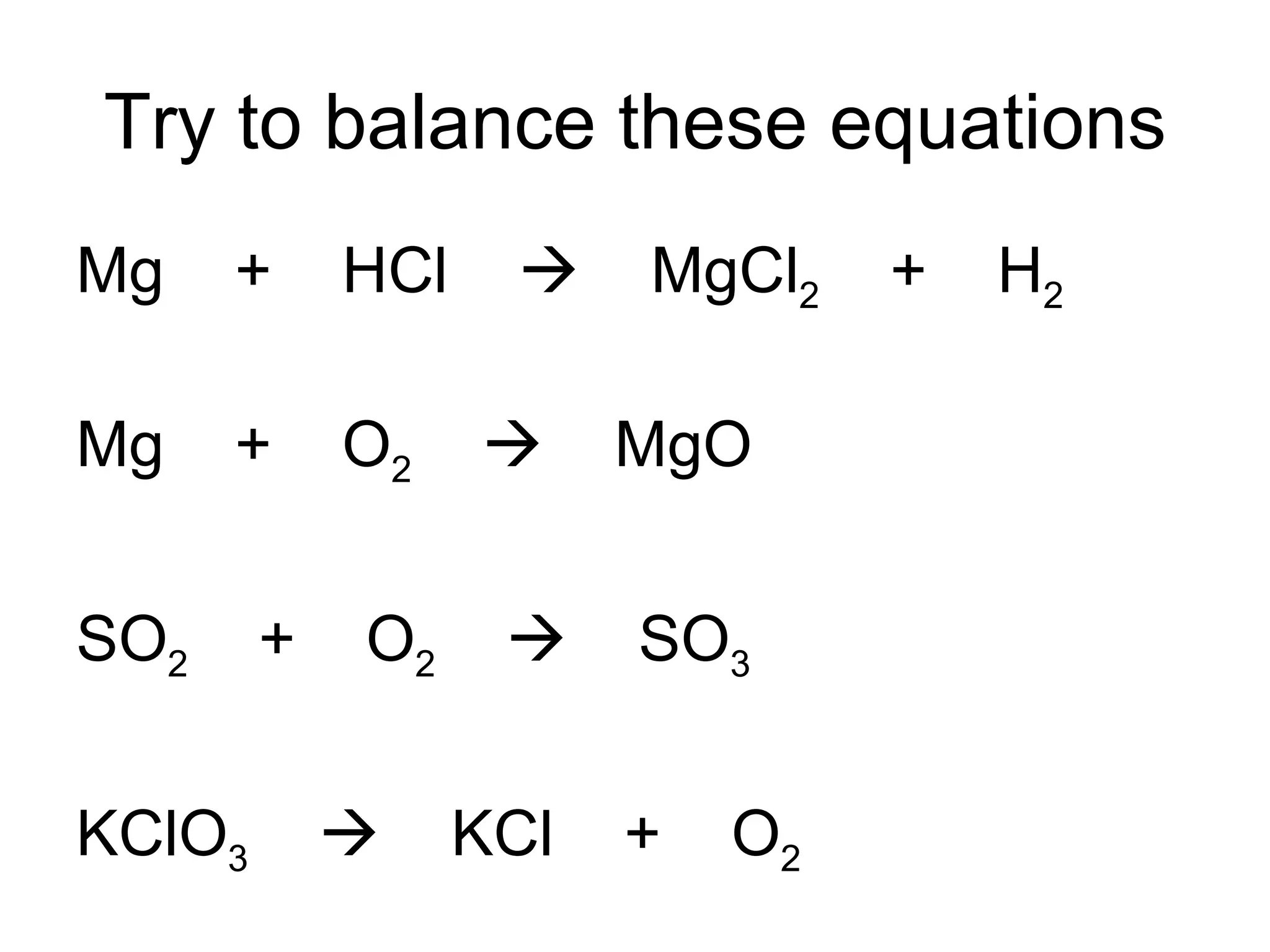
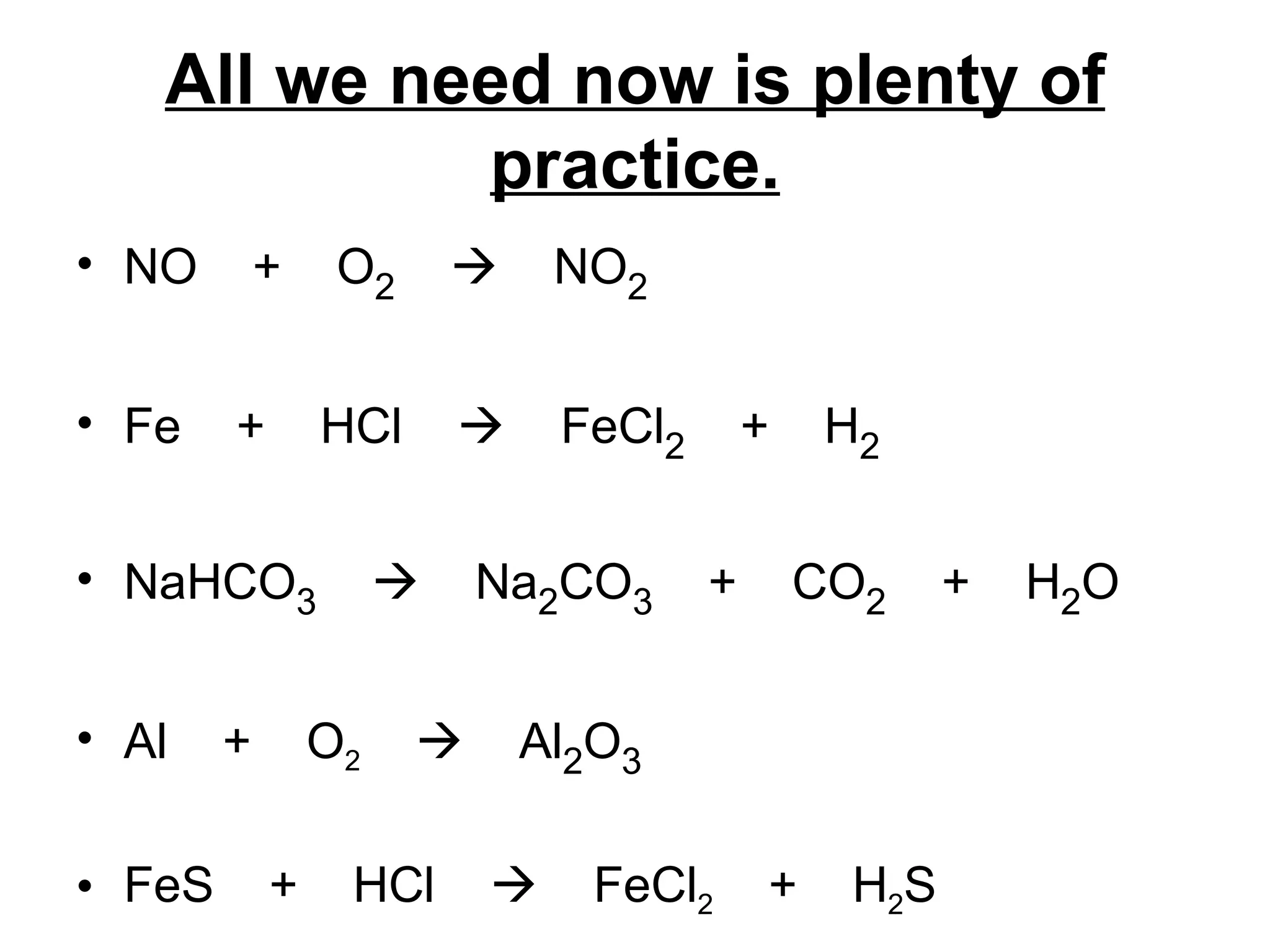
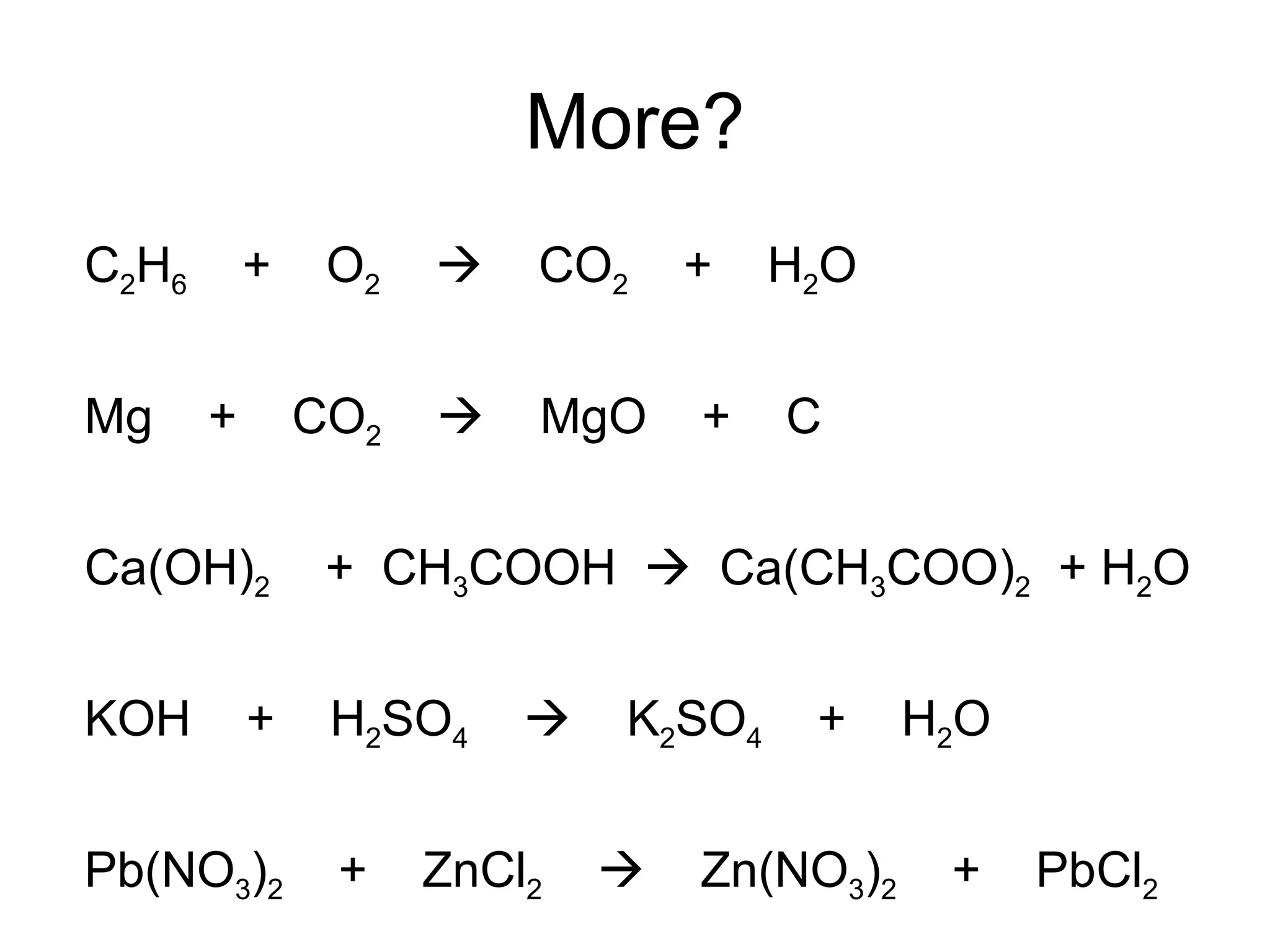
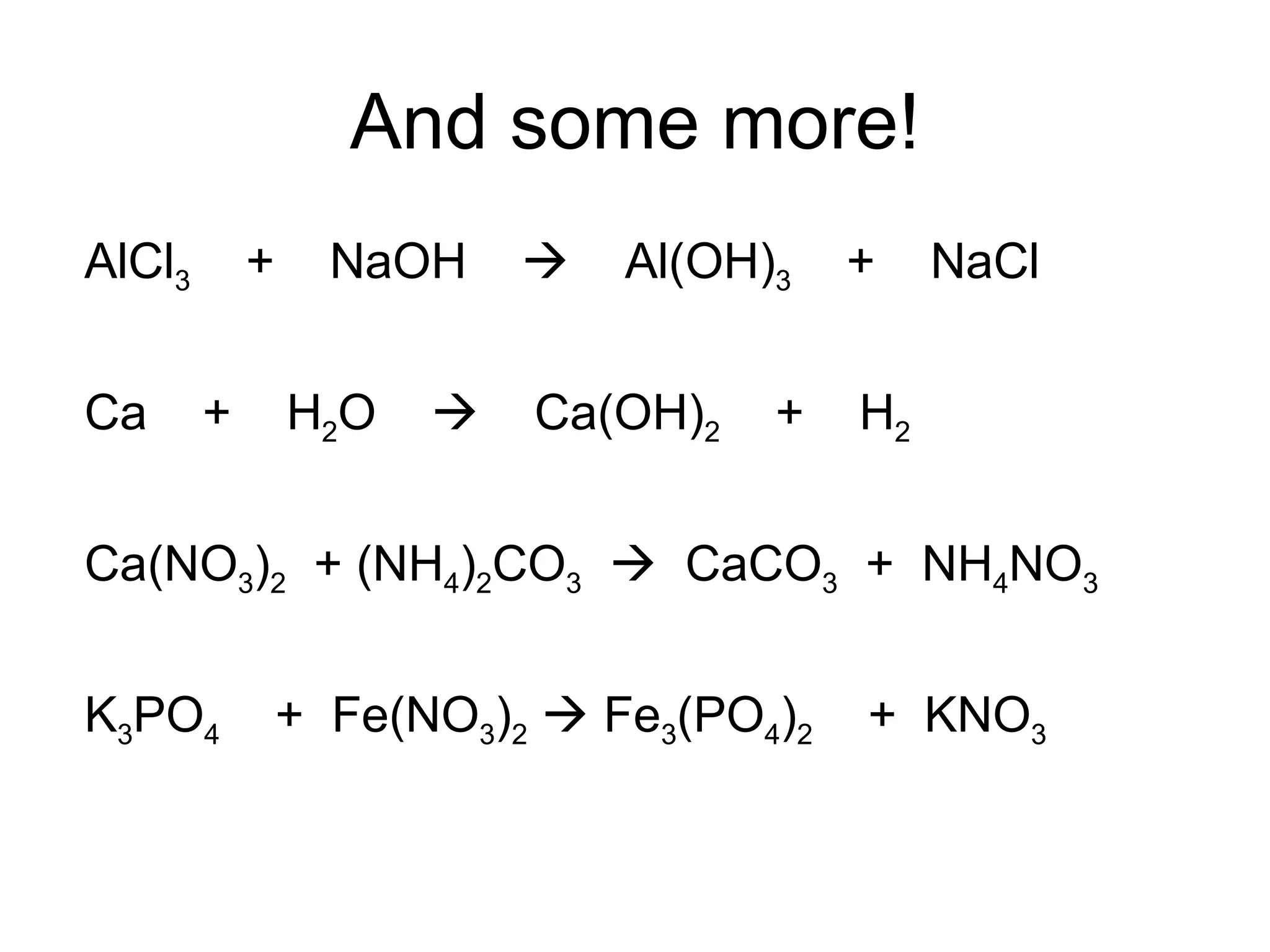
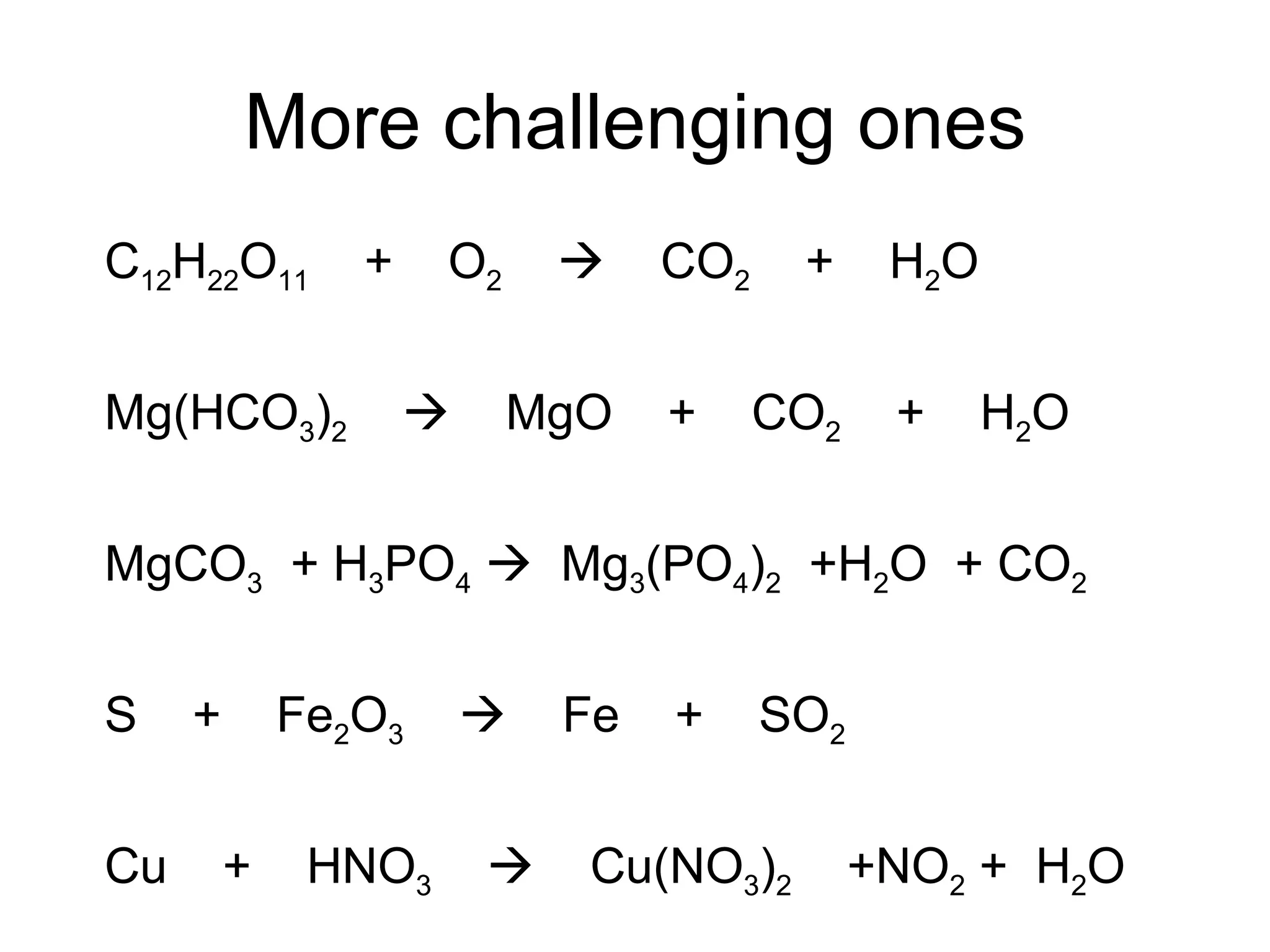
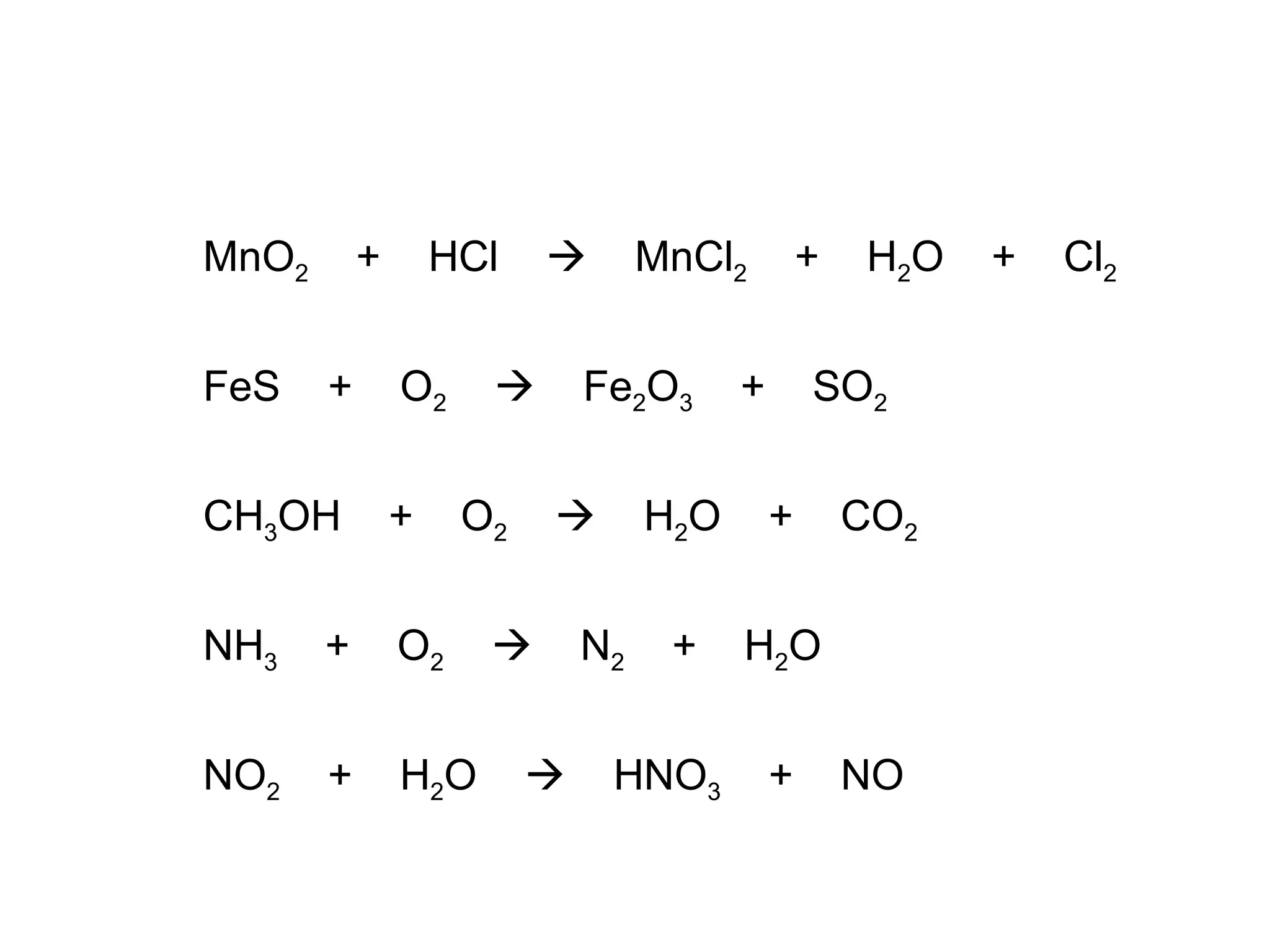
The document discusses balancing chemical equations by ensuring that the same number and type of atoms appear on both sides of the reaction arrow. It provides examples of balancing a variety of chemical equations involving reactants like Mg, HCl, SO2, and products like MgCl2, SO3, NO2. Students are encouraged to practice balancing many different types of equations involving metals, nonmetals, acids, bases, and other chemicals.