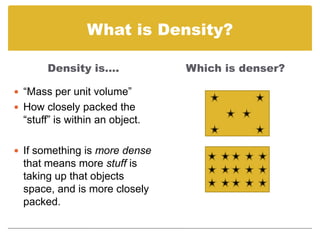
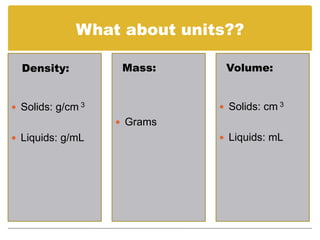
Density is defined as mass per unit volume. It describes how closely packed the molecules of a substance are. Density can be used to calculate mass or volume when two of the three properties are known using the formula: Density = Mass/Volume, Mass = Density x Volume, or Volume = Mass/Density. Substances with a higher density than water (1 g/mL) will sink, while those with a lower density will float.