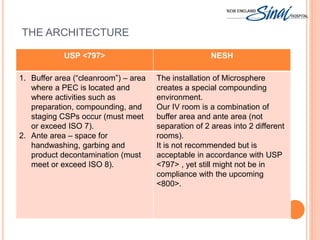
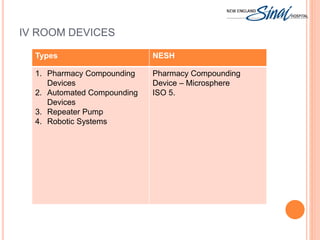
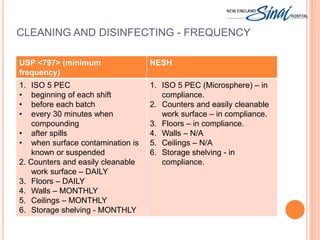
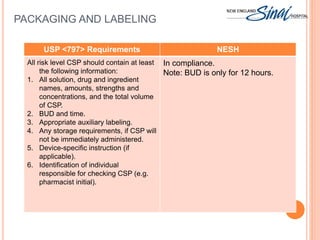
The document summarizes the requirements for an IV room project based on USP <797> standards. It discusses the primary engineering controls including laminar airflow workbenches and biological safety cabinets. It also outlines the architecture of buffer and ante areas, recommended devices for compounding, cleaning and disinfecting frequency guidelines, personnel hygiene and garbing procedures, packaging and labeling standards, and staff responsibilities. The IV room is designed to meet most USP requirements, with some exceptions like not fully separating the buffer and ante areas into different rooms.