Embed presentation
Downloaded 10 times

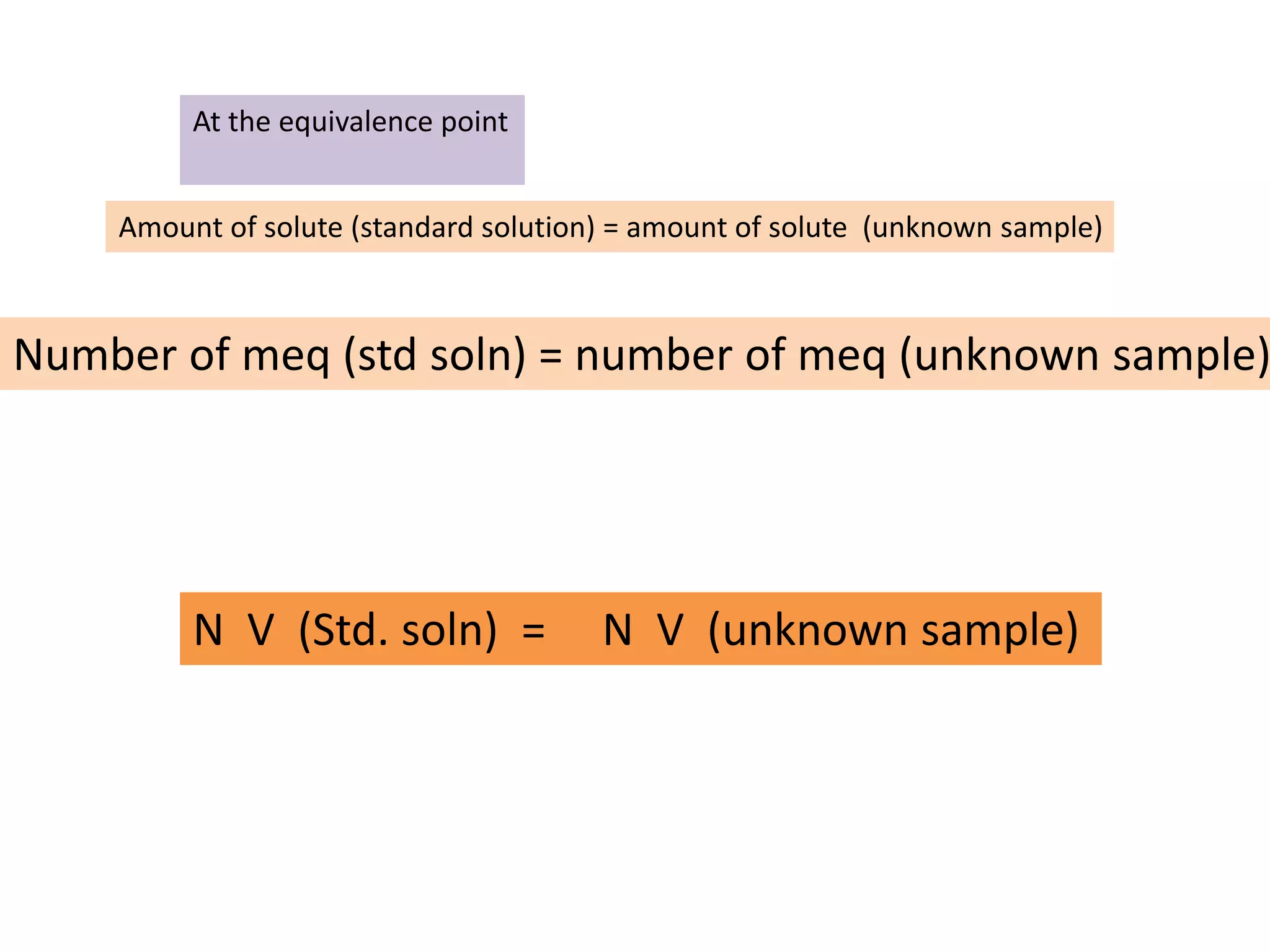


Titration is a process used to determine the concentration of an unknown substance. It involves adding a measured volume of the unknown sample and titrating it with a standard solution until the equivalence point is reached. The equivalence point is identified using an indicator that changes color at the exact point when the amounts of reacting substances are equivalent. The volume of standard solution used is then used to calculate the concentration of the original unknown sample.


