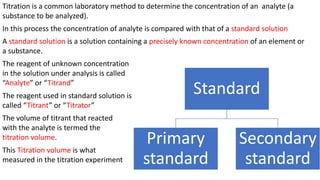
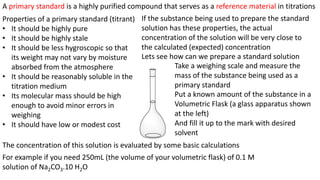
Titration is a common laboratory technique used to determine the concentration of an unknown analyte solution. It involves titrating the analyte with a standard solution of known concentration. The volume of standard solution required to reach the endpoint of the titration reaction is used to calculate the concentration of the analyte based on stoichiometric ratios between the reactants. Preparing an accurate primary standard solution involves precisely weighing and dissolving a purified compound in a volumetric flask and filling to the mark to obtain a solution of known molarity. This standard can then be used to titrate analytes and determine their concentrations.