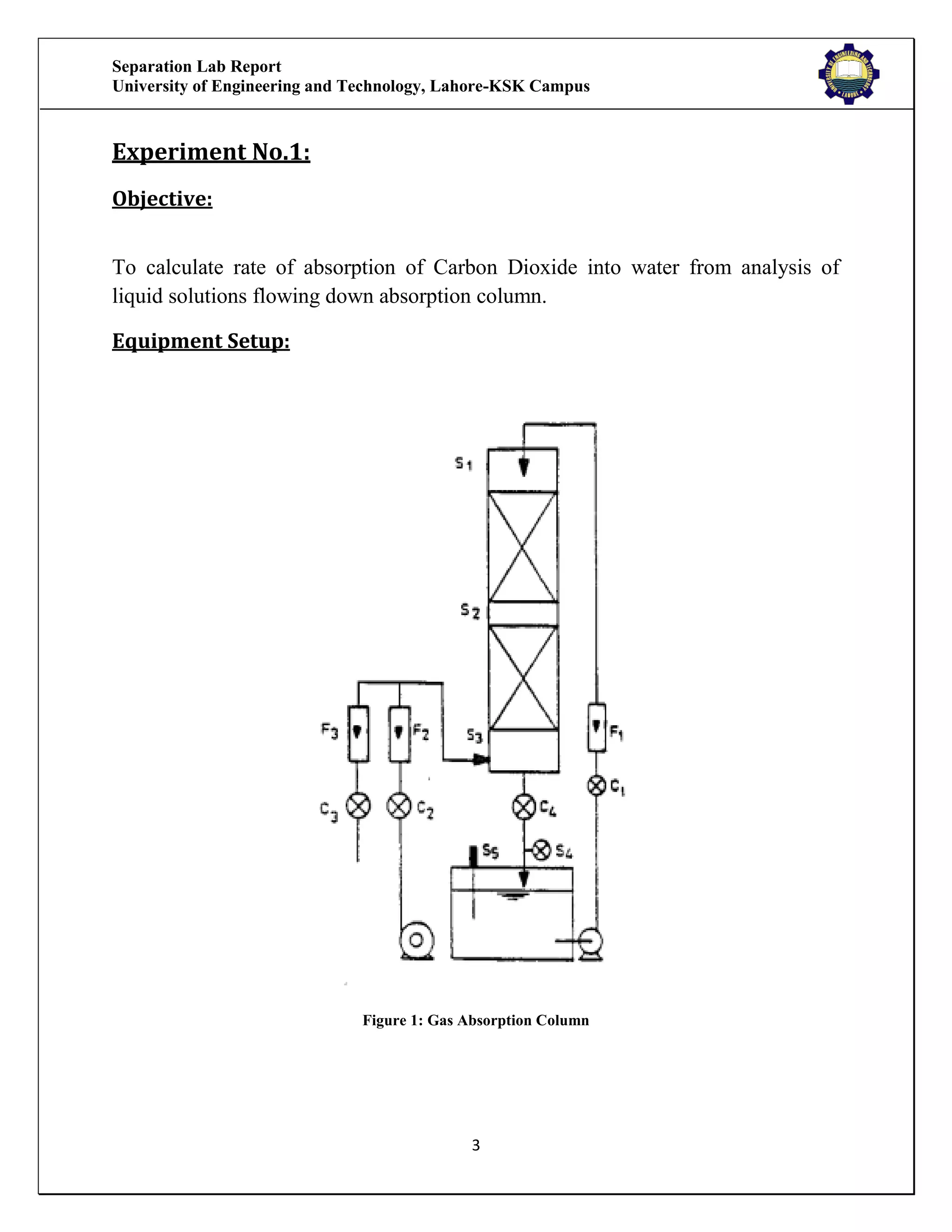
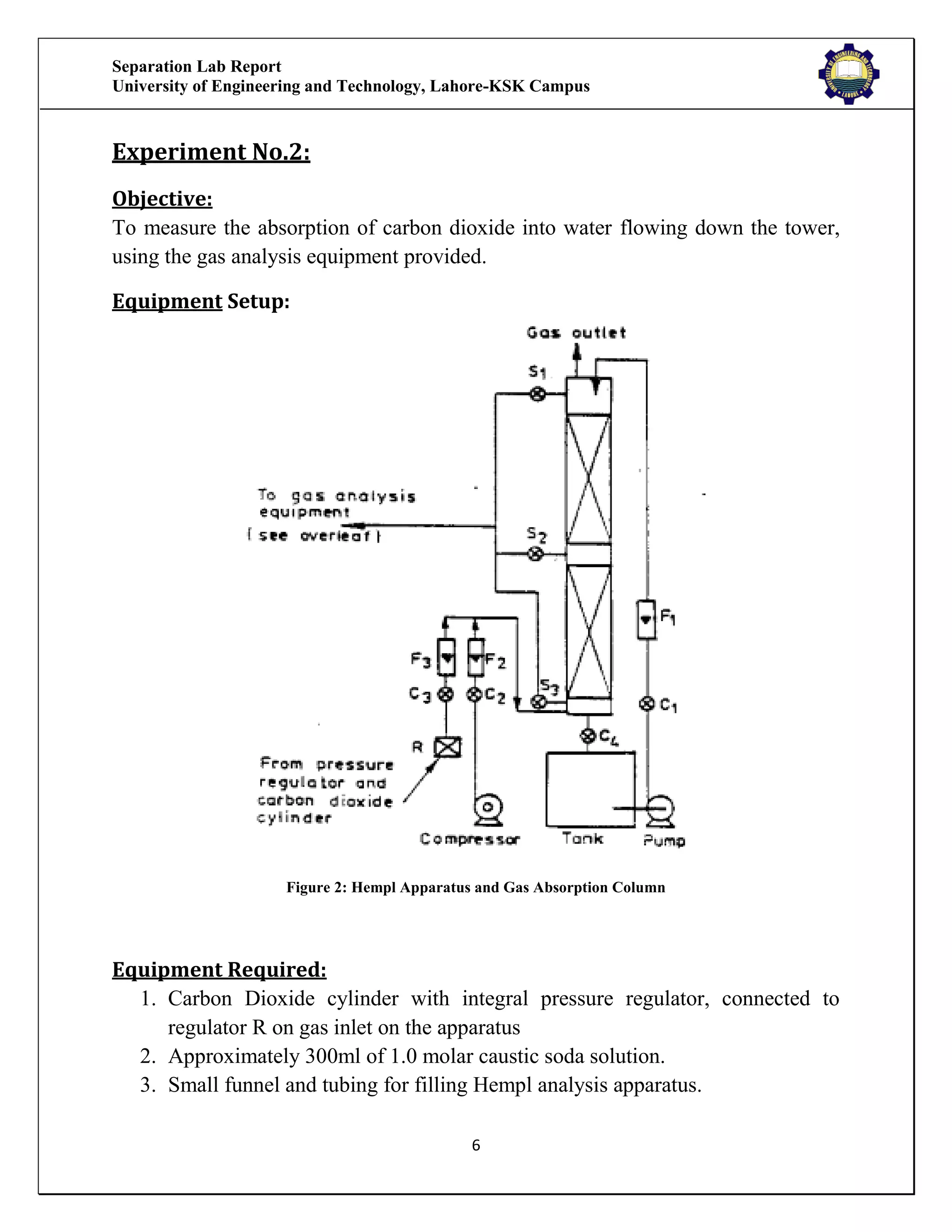
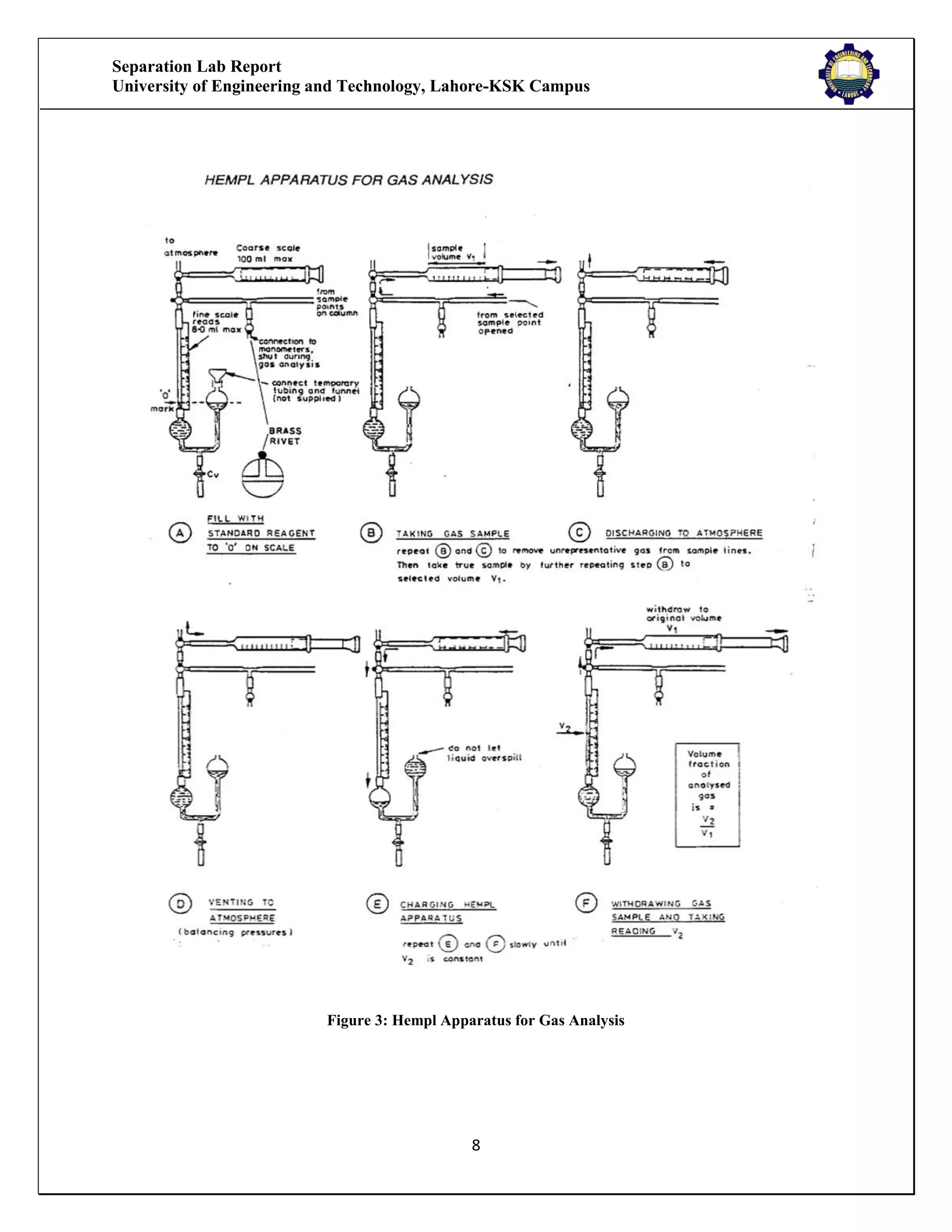
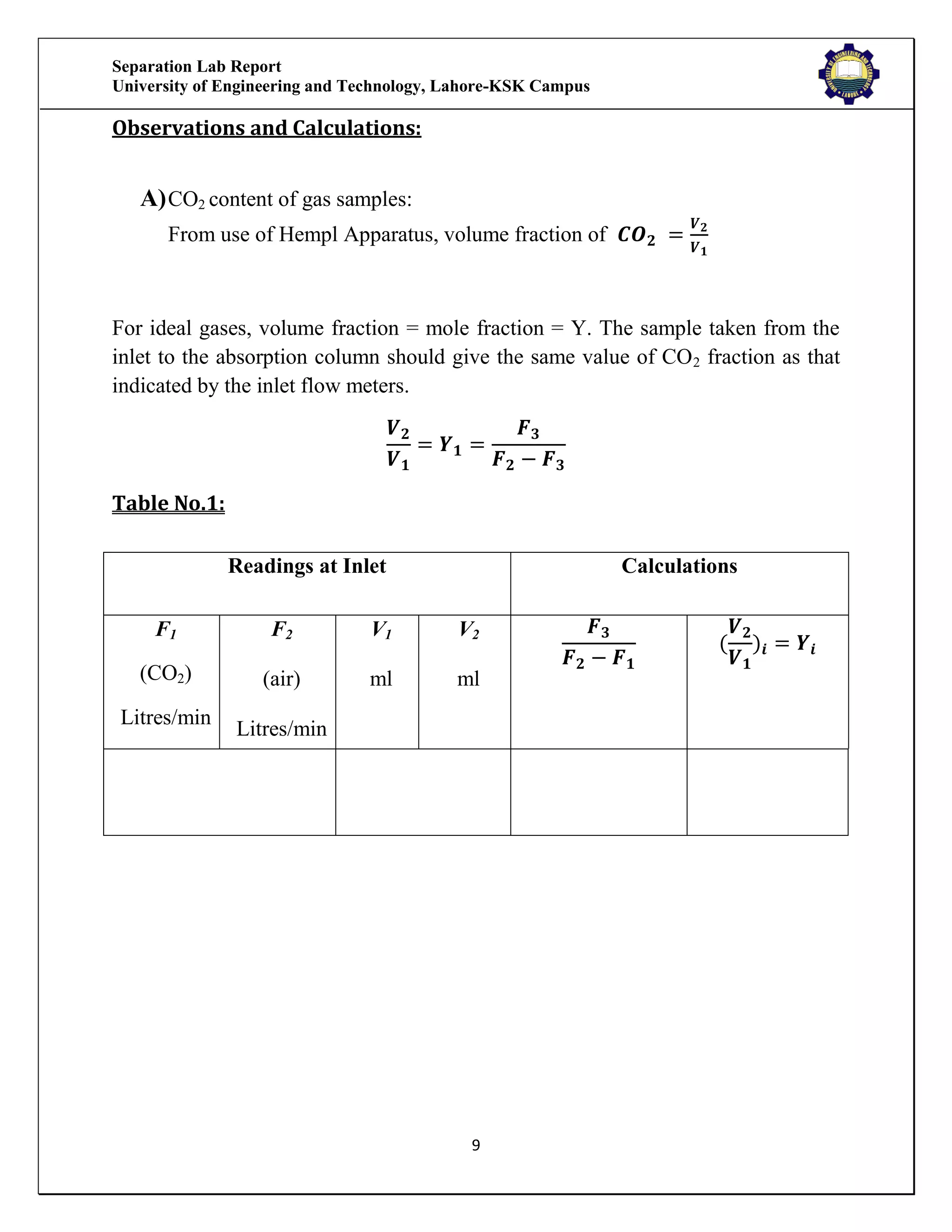
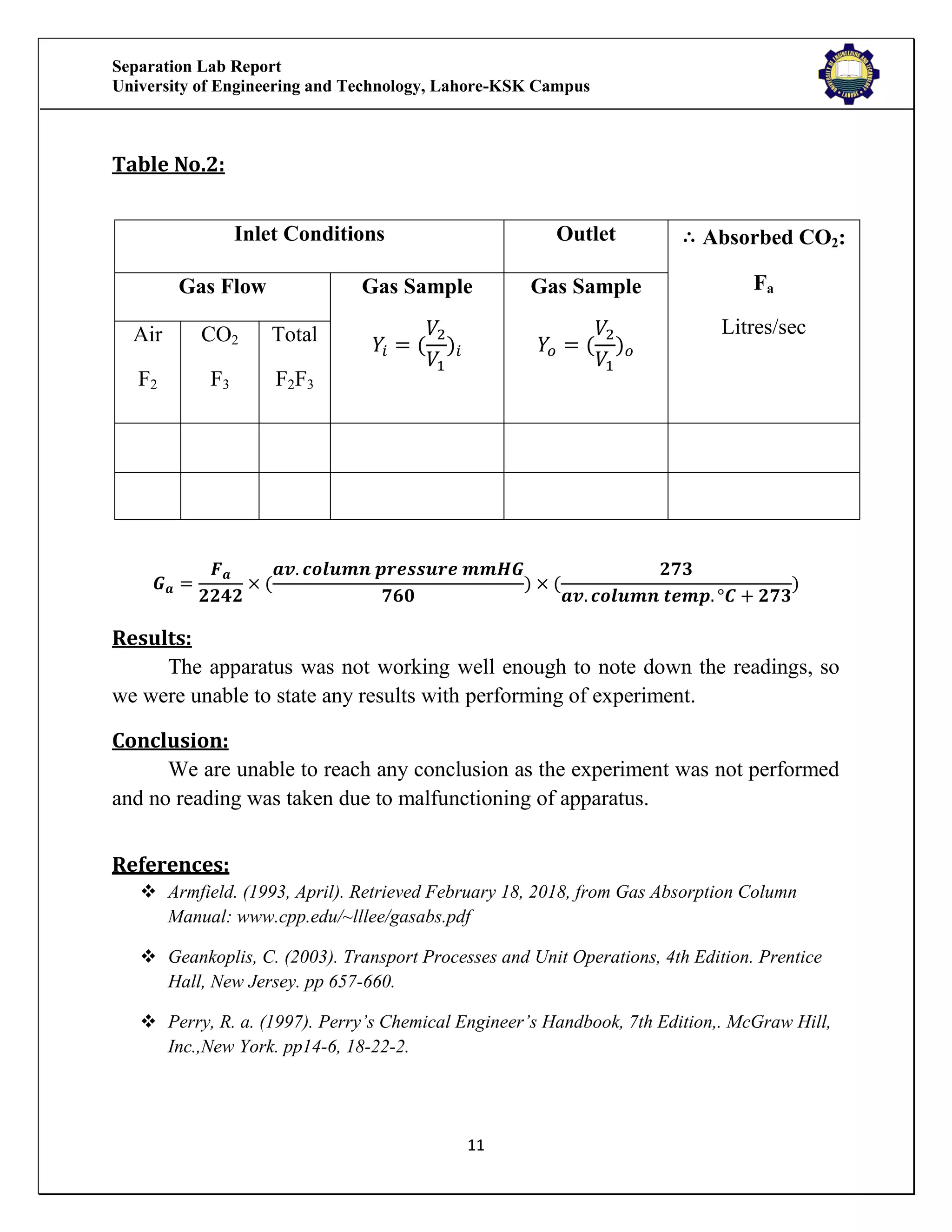
The separation lab report from the University of Engineering and Technology, Lahore-KSK Campus, details two experiments focusing on gas absorption using a gas absorption column to analyze the absorption of CO2 in water. The first experiment successfully demonstrates the process and relationship between the absorption rate and time, while the second experiment could not be concluded due to equipment malfunction. Both experiments aim to train students in practical applications and industrial usages of gas absorption techniques.