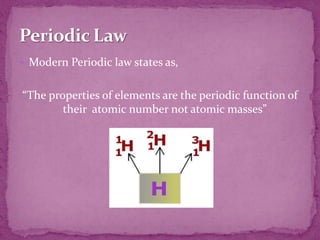
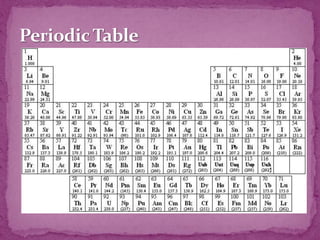
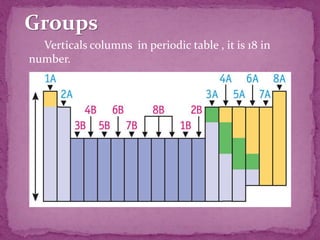
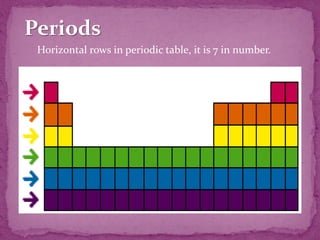
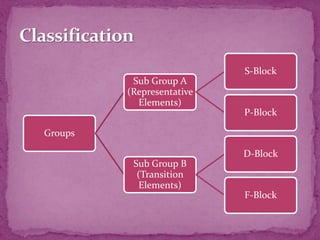
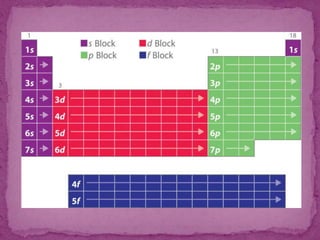
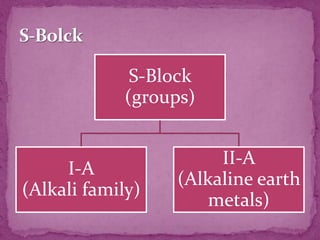
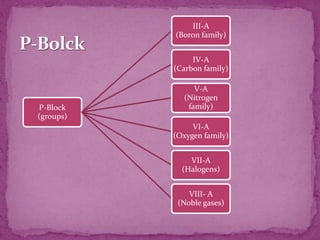
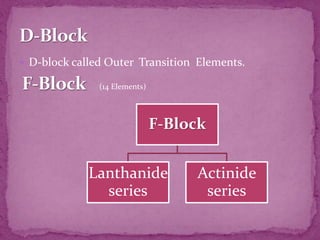
The document discusses the history and development of the periodic table. It explains that Mendeleev was the first to publish a periodic table in 1869, organizing elements based on atomic mass and recognizing that elements with similar properties fell into vertical columns. However, Mendeleev's table had some anomalies, like incorrect placement of some elements. Moseley later proposed using atomic number instead of atomic mass, solving these anomalies. The modern periodic table is organized into periods and groups based on atomic number, with blocks for different orbital types. It provides information about elemental properties and reactions.