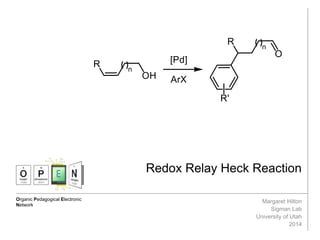
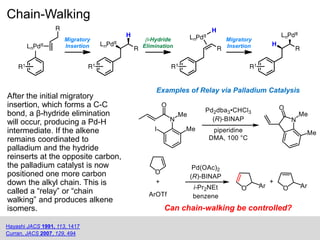
This document summarizes work on the Heck reaction and redox-relay Heck reaction. It provides background on the Heck reaction and its applications. It then discusses chain-walking in the Heck reaction where the palladium catalyst migrates down an alkyl chain, producing alkene isomers. It introduces Sigman's work developing a redox-relay Heck reaction where chain-walking of palladium is controlled by an alcohol thermodynamic sink on the substrate, transferring unsaturation to form aldehydes or ketones. It is authored by Margaret Hilton from the Sigman Lab at the University of Utah in 2014.