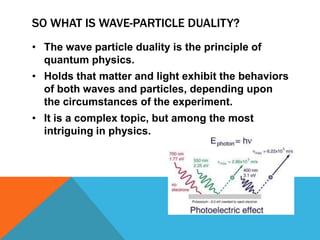
Wave-particle duality proposes that all particles exhibit both wave and particle properties. Light was originally thought to consist solely of waves or particles, but through the work of Planck, Einstein, Bohr and others, it is now understood that particles also have a wave nature, and vice versa. This phenomenon of wave-particle duality is a fundamental principle of quantum physics where matter and light behave as both waves and particles depending on the experimental conditions.