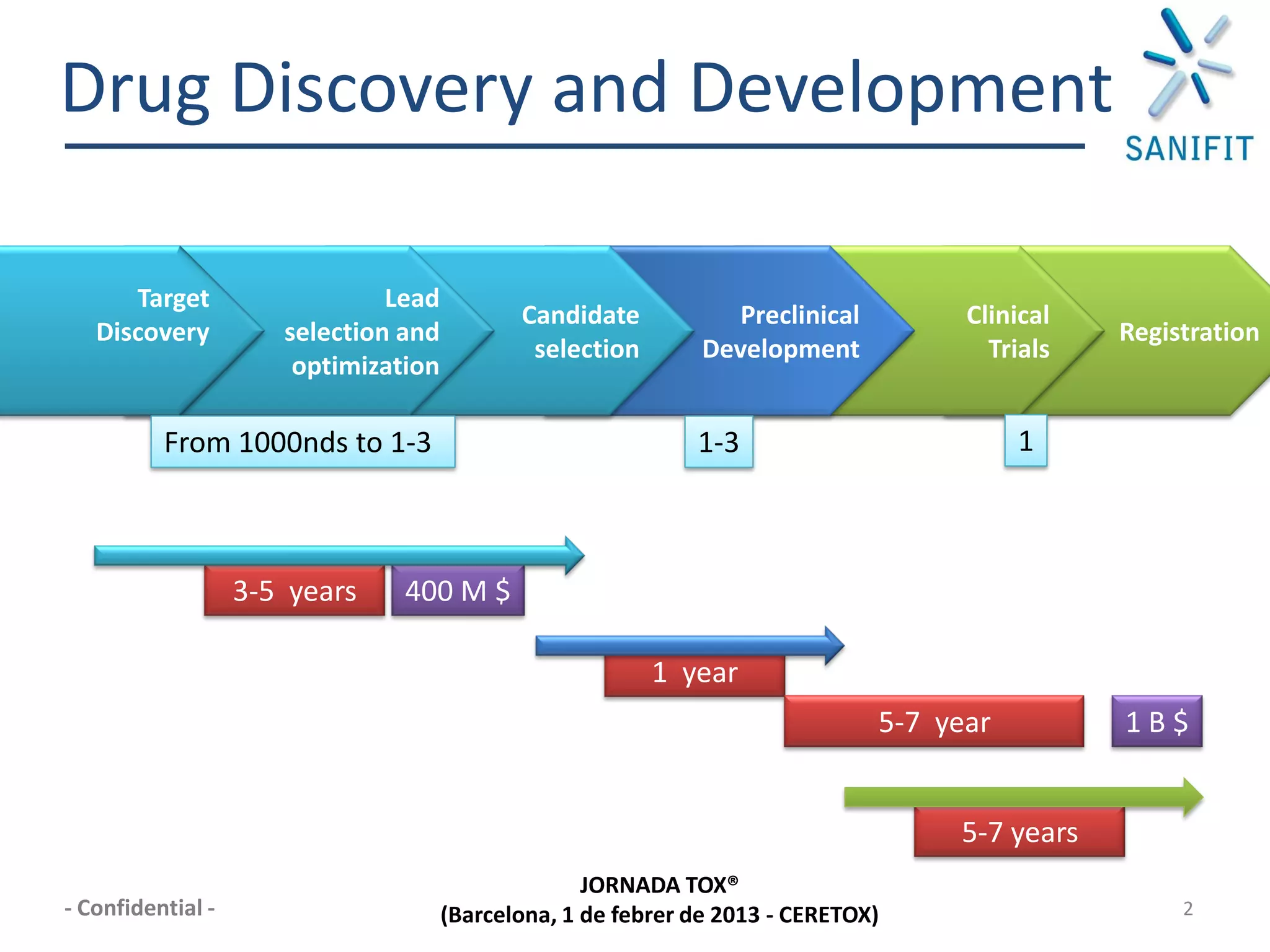
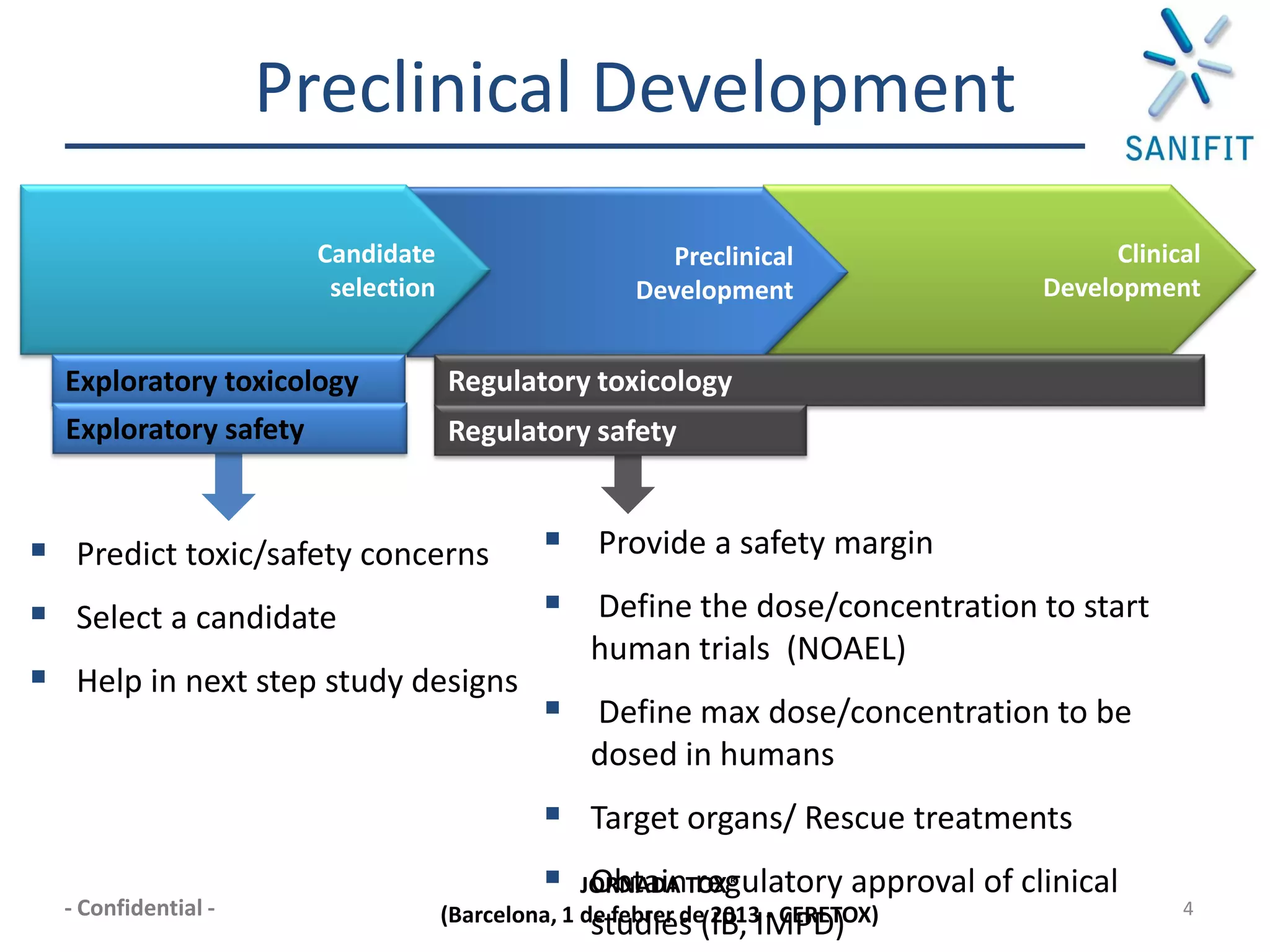
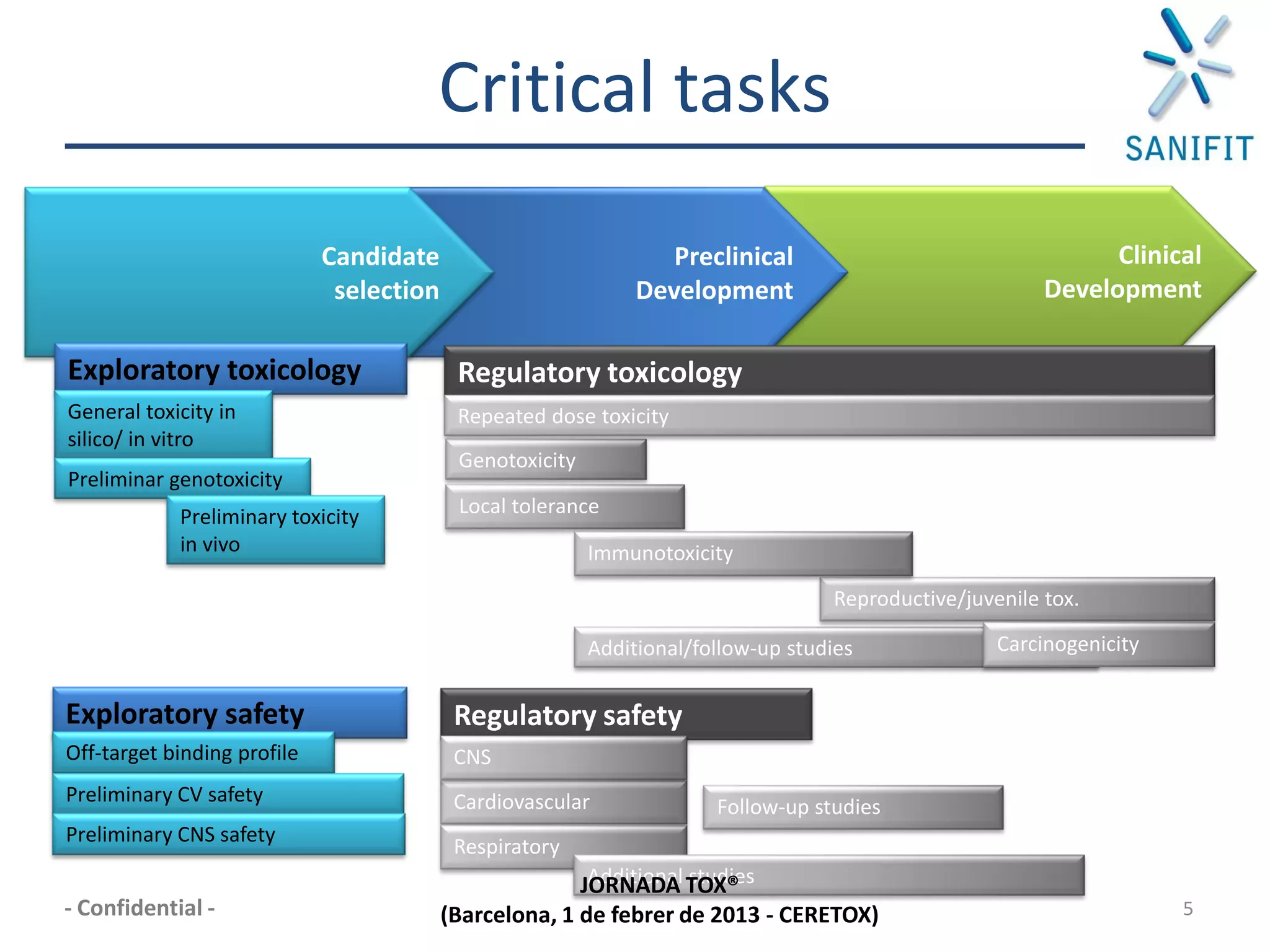
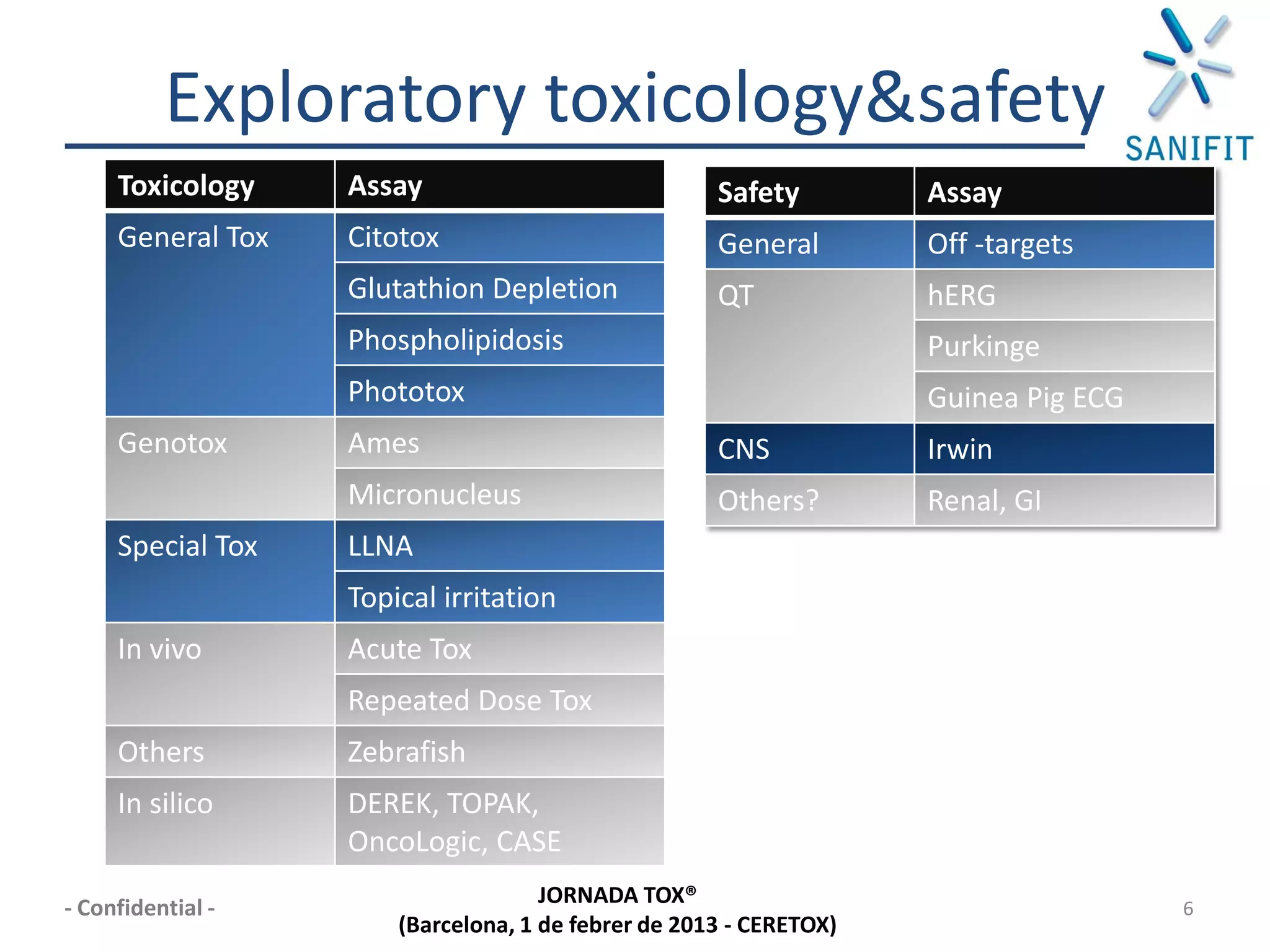
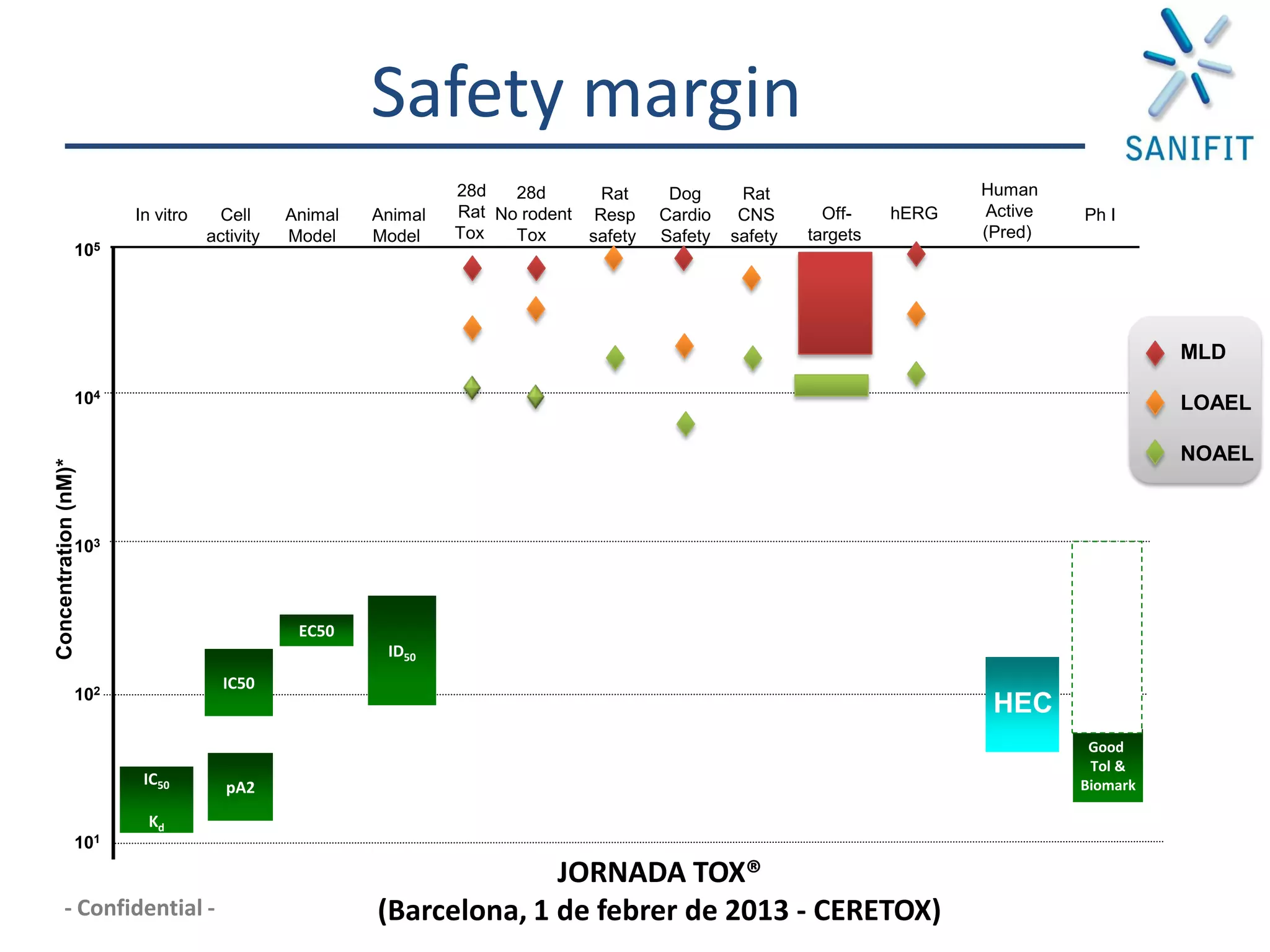
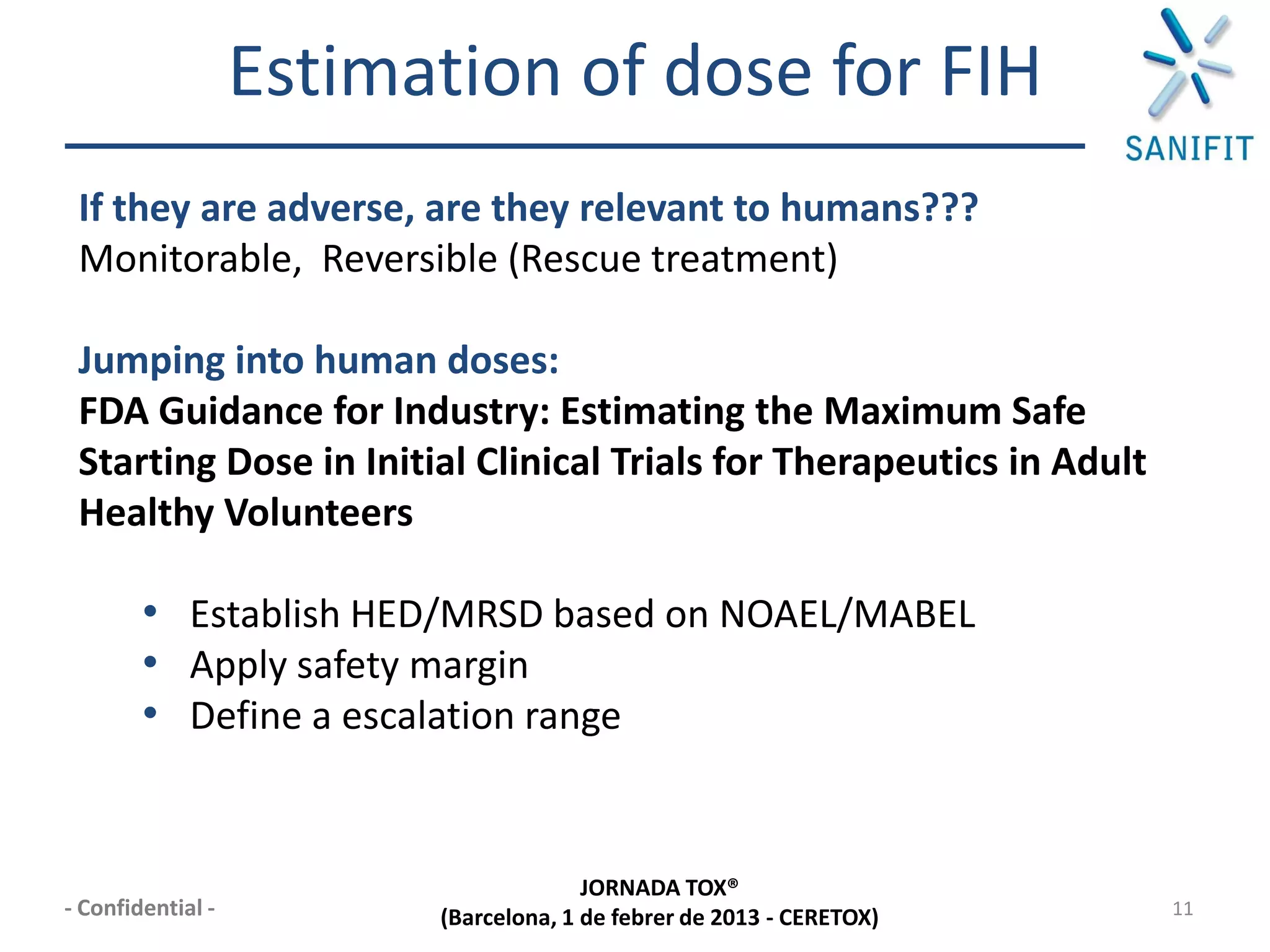
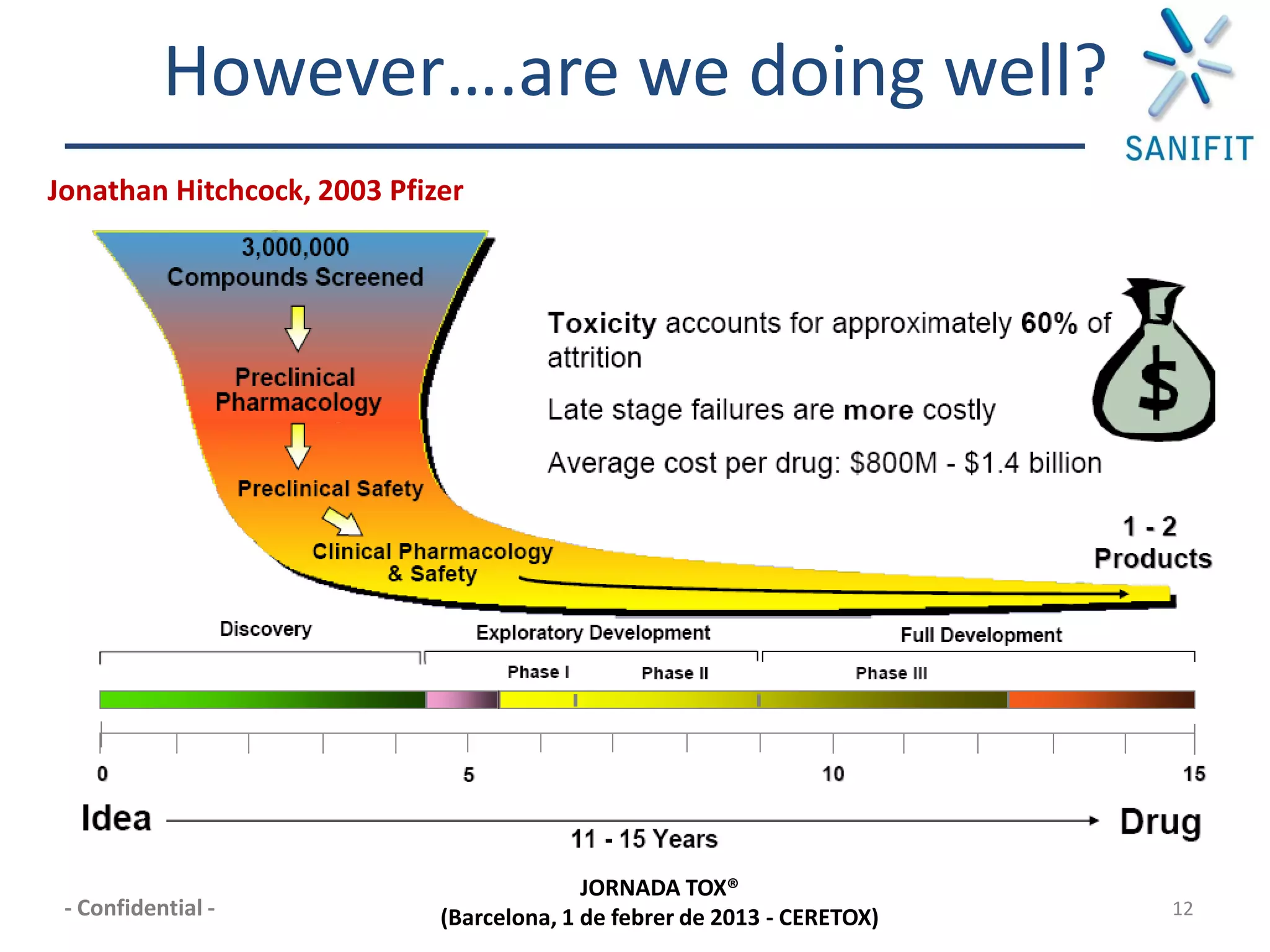
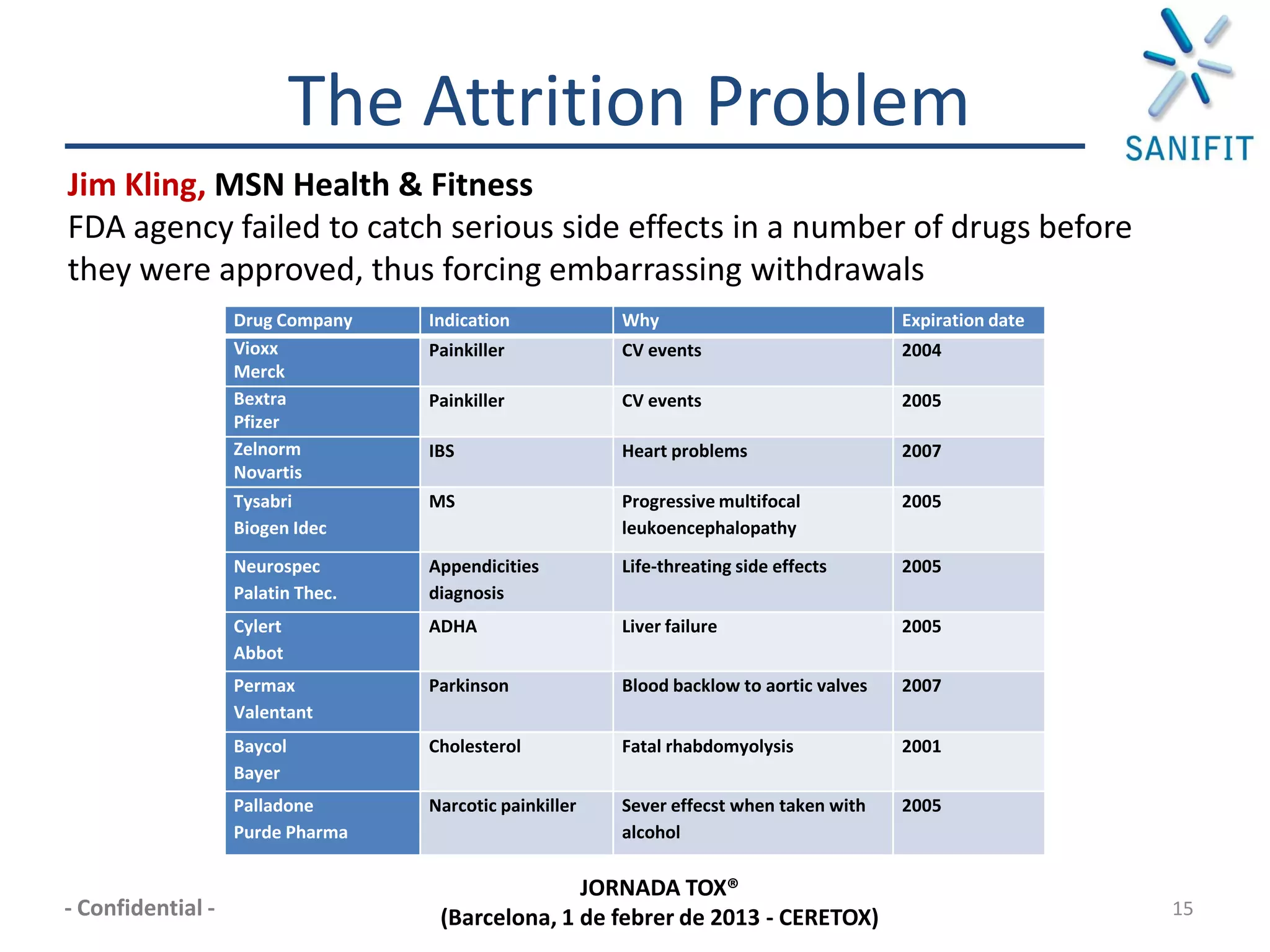
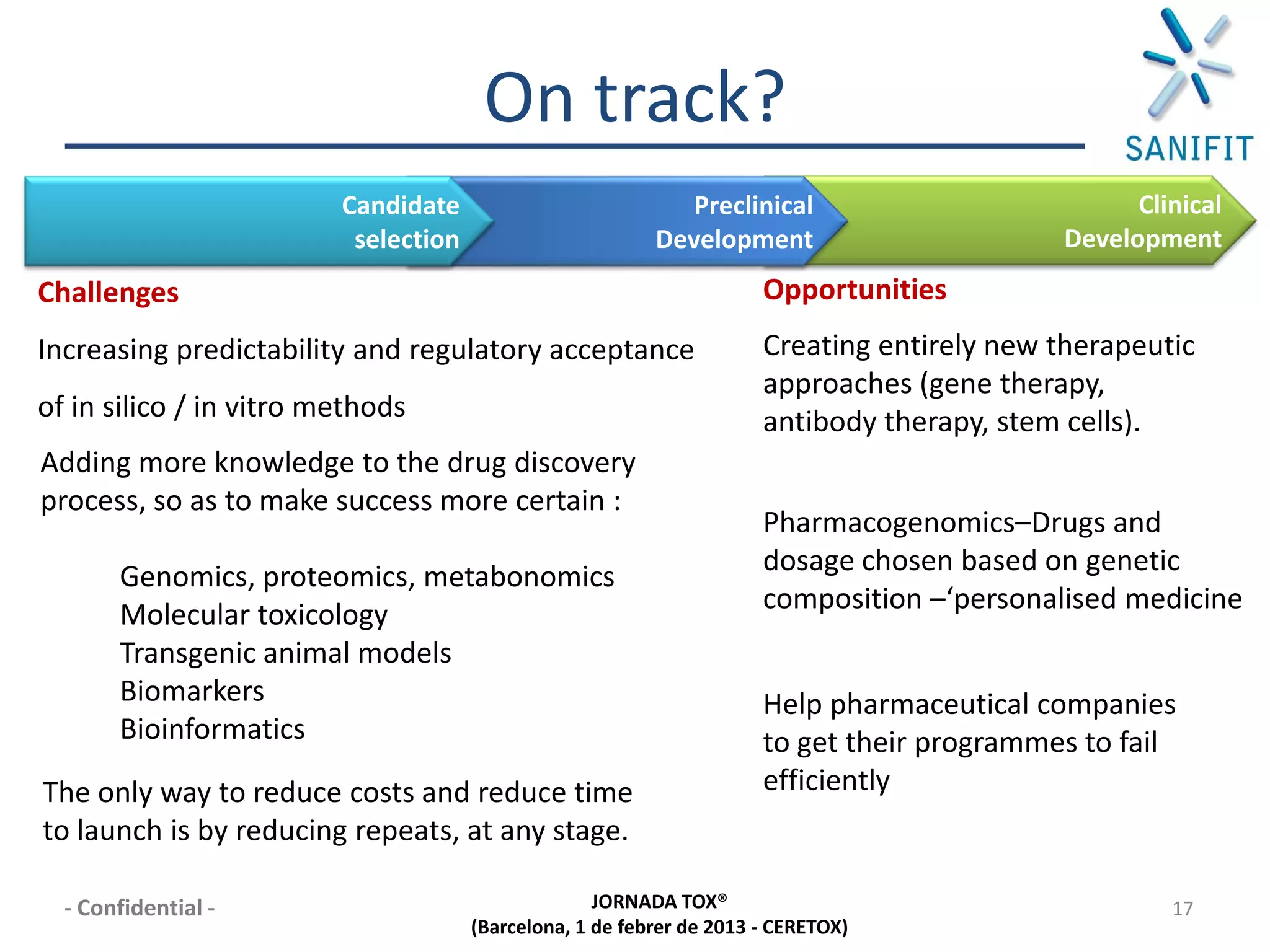
The document discusses preclinical development and whether changes are needed. It outlines the stages from target discovery to registration, noting the high costs and attrition rates. Exploratory toxicology aims to predict toxicities and select candidates, while regulatory toxicology provides safety margins for starting human trials. A variety of assays are used to evaluate general toxicity, genotoxicity, safety pharmacology and more. Challenges include poor translation between preclinical models and humans. Opportunities exist to improve prediction through new technologies and approaches. Overall, advances have been made but high attrition rates continue to motivate further improvements.