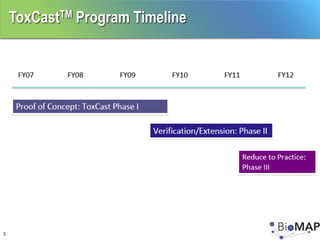
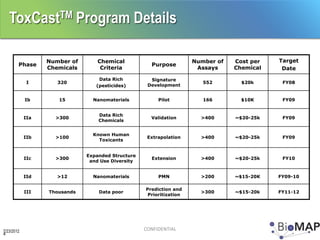
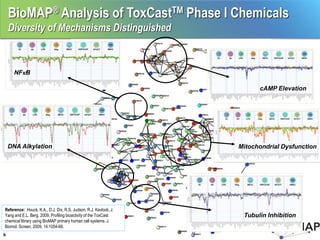
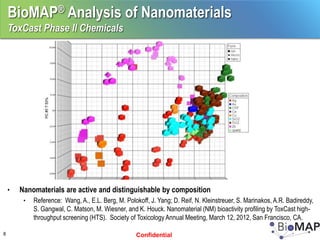
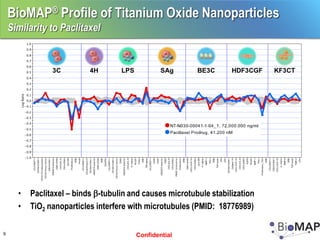
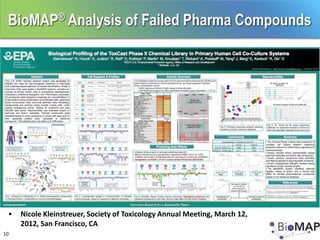
The EPA ToxCast program, with BioSeek as a contractor since 2007, focuses on a cost-effective approach for toxicity testing of thousands of chemicals through three phases: proof-of-concept, verification, and practical application. The program has developed a variety of data-rich signatures and has publicly posted its findings. The document further details biomap analysis outcomes, nanomaterial bioactivity, and related publications and presentations.