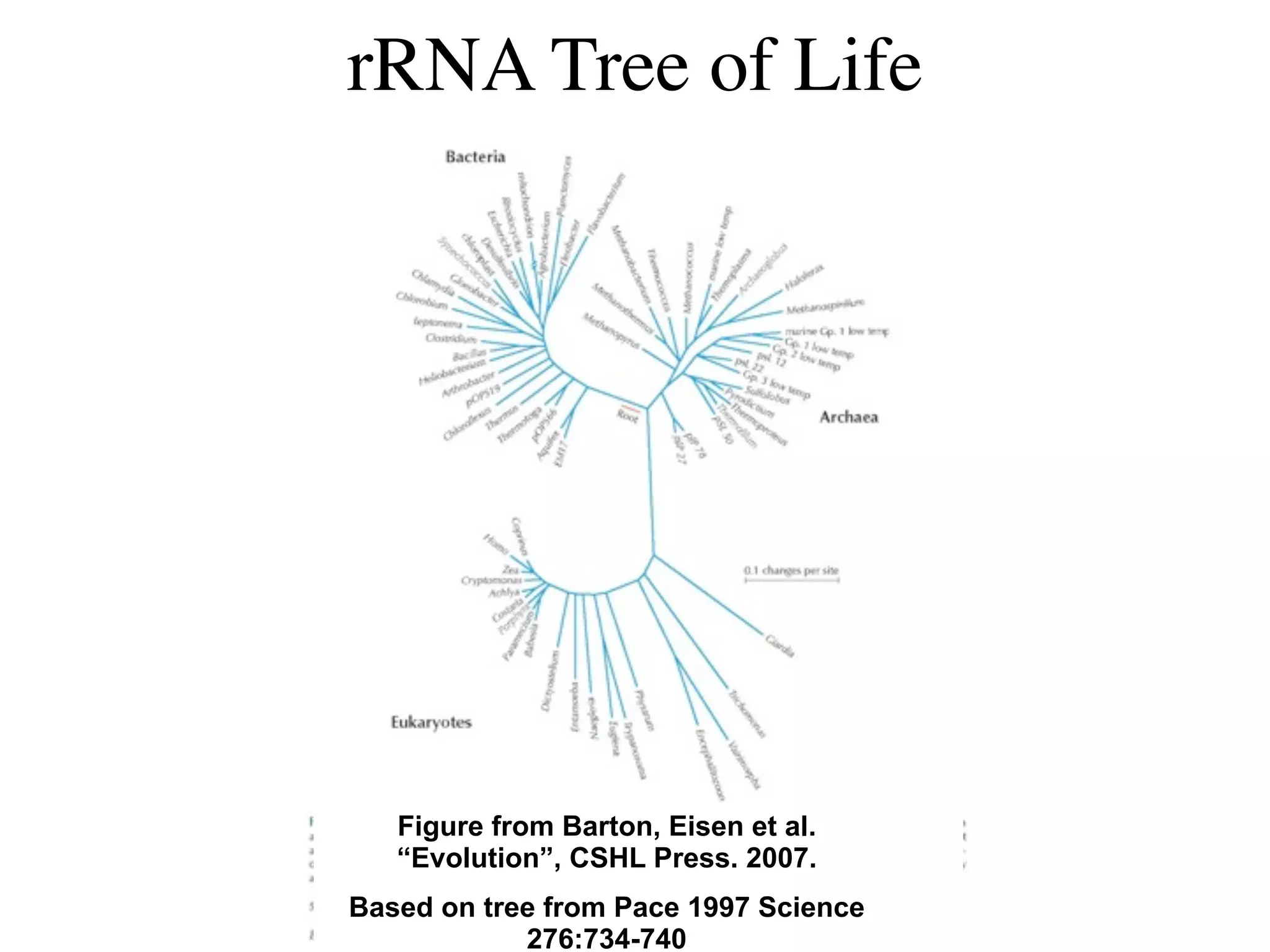
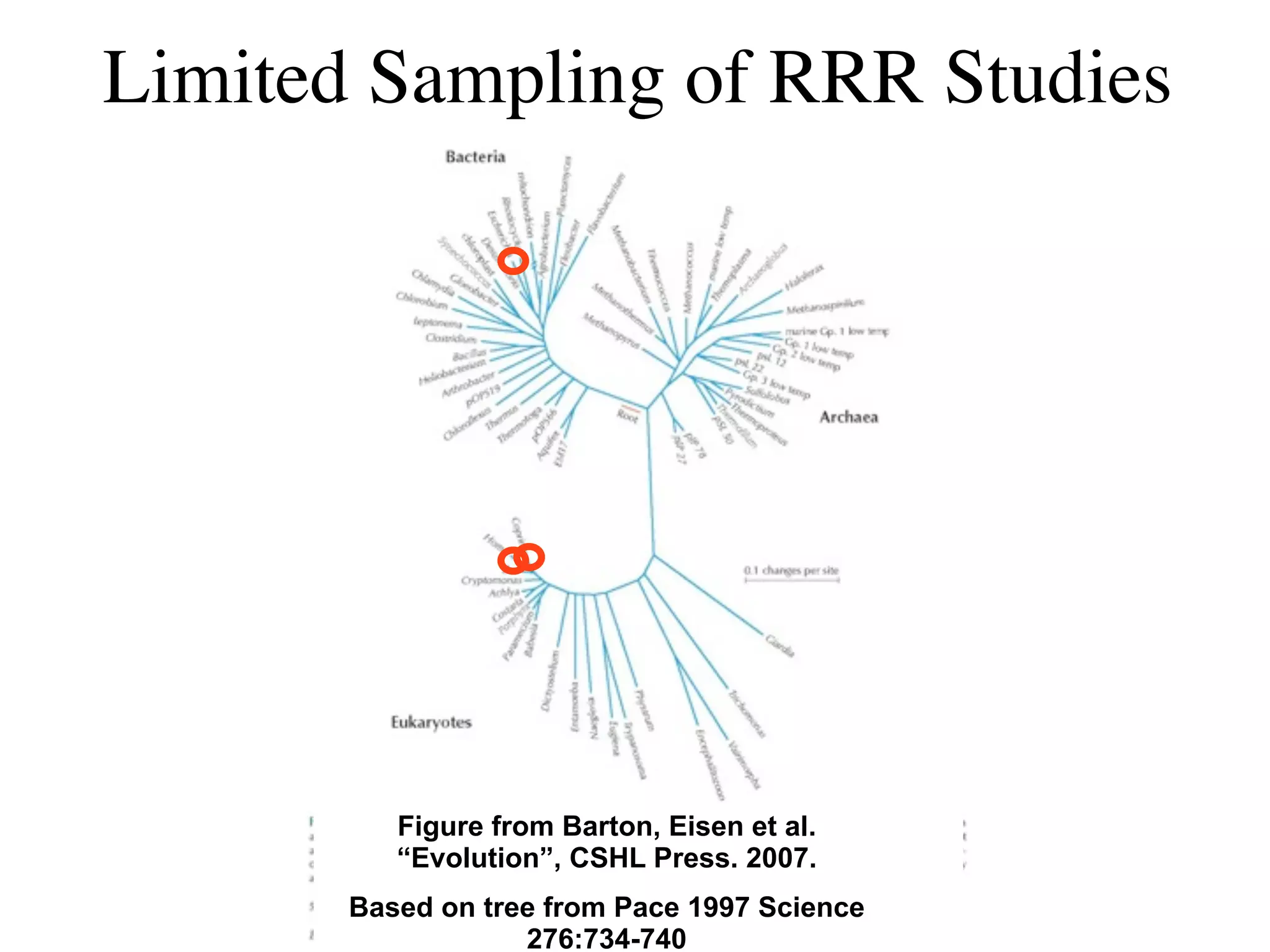
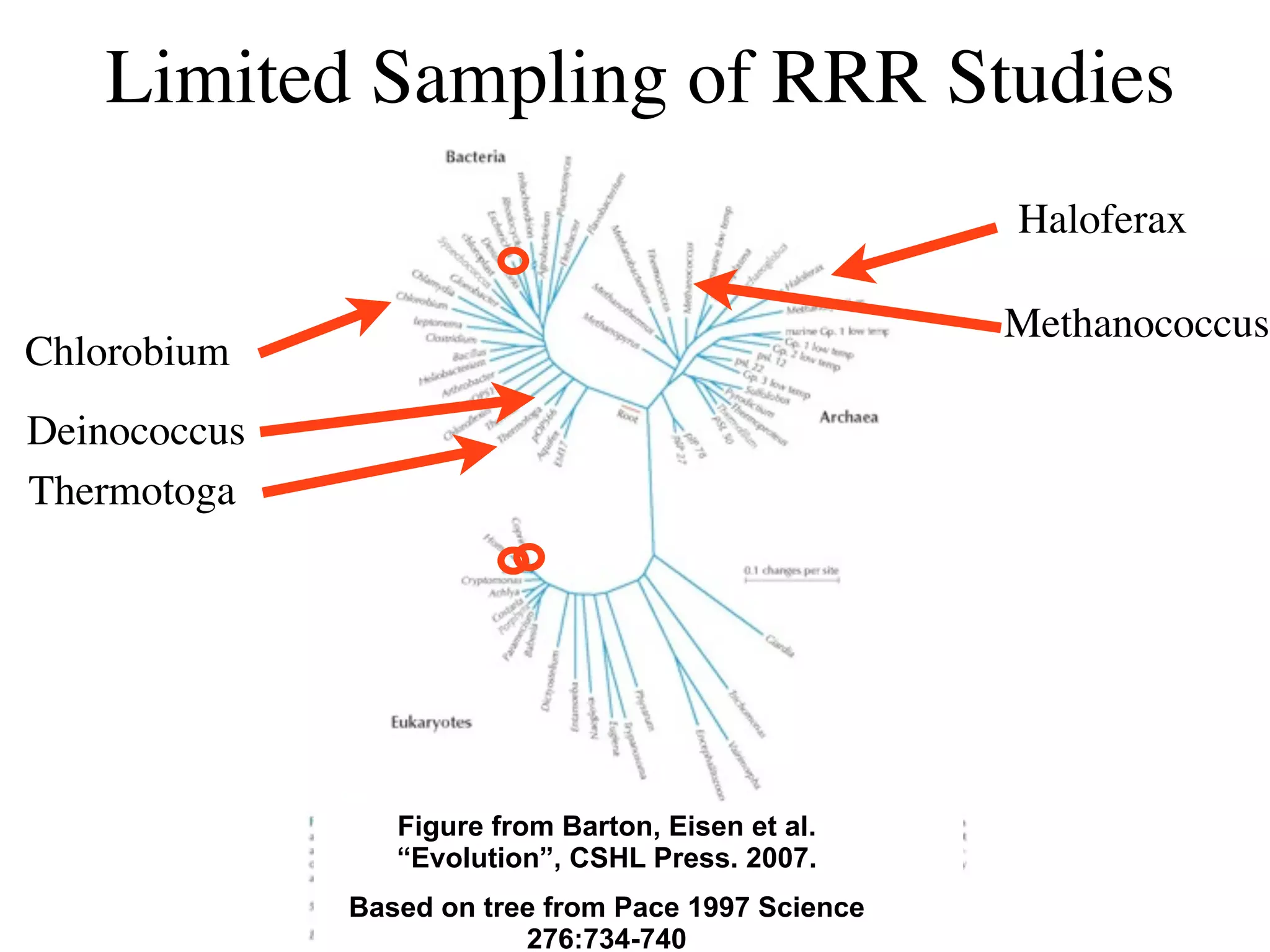
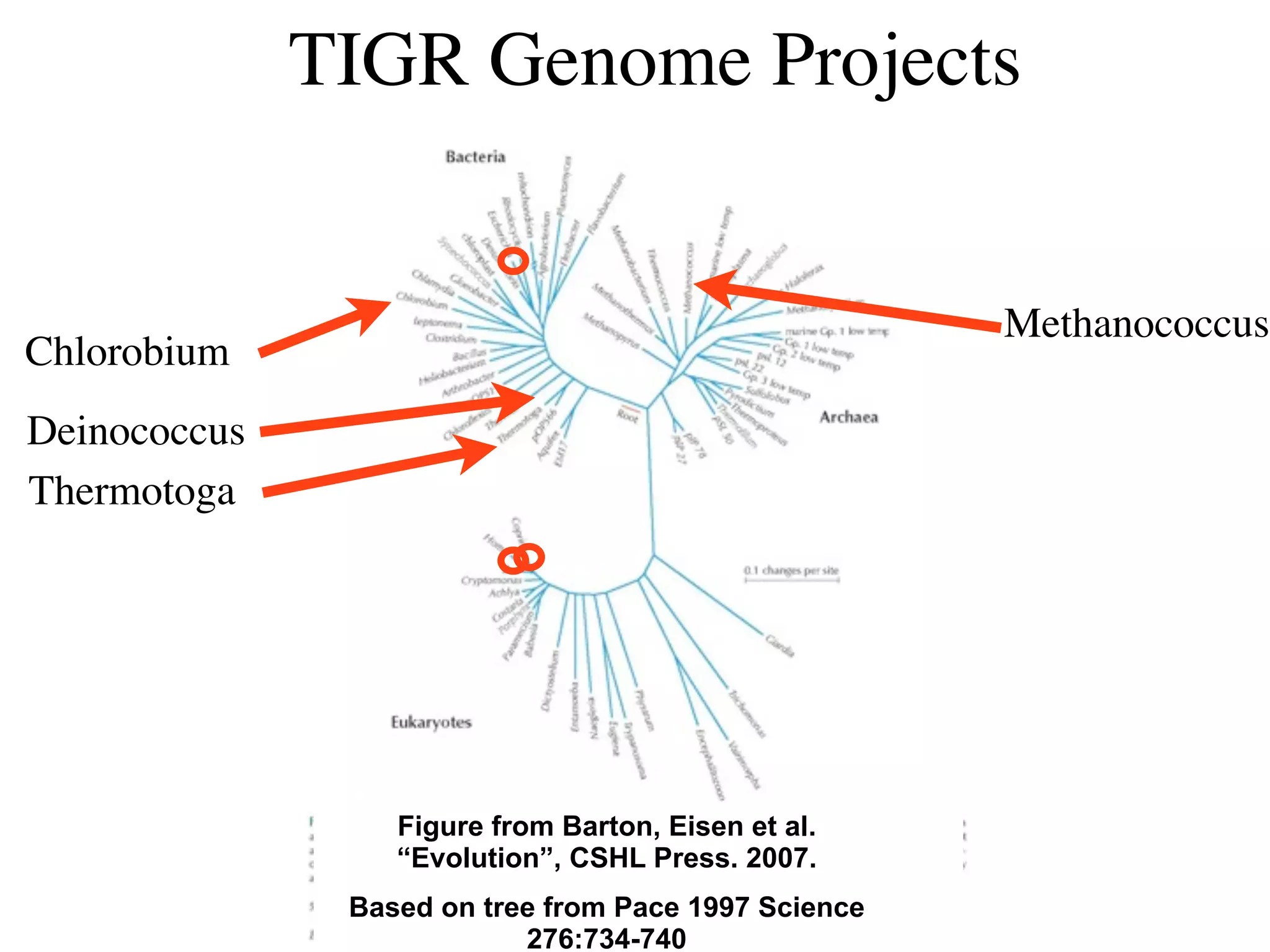
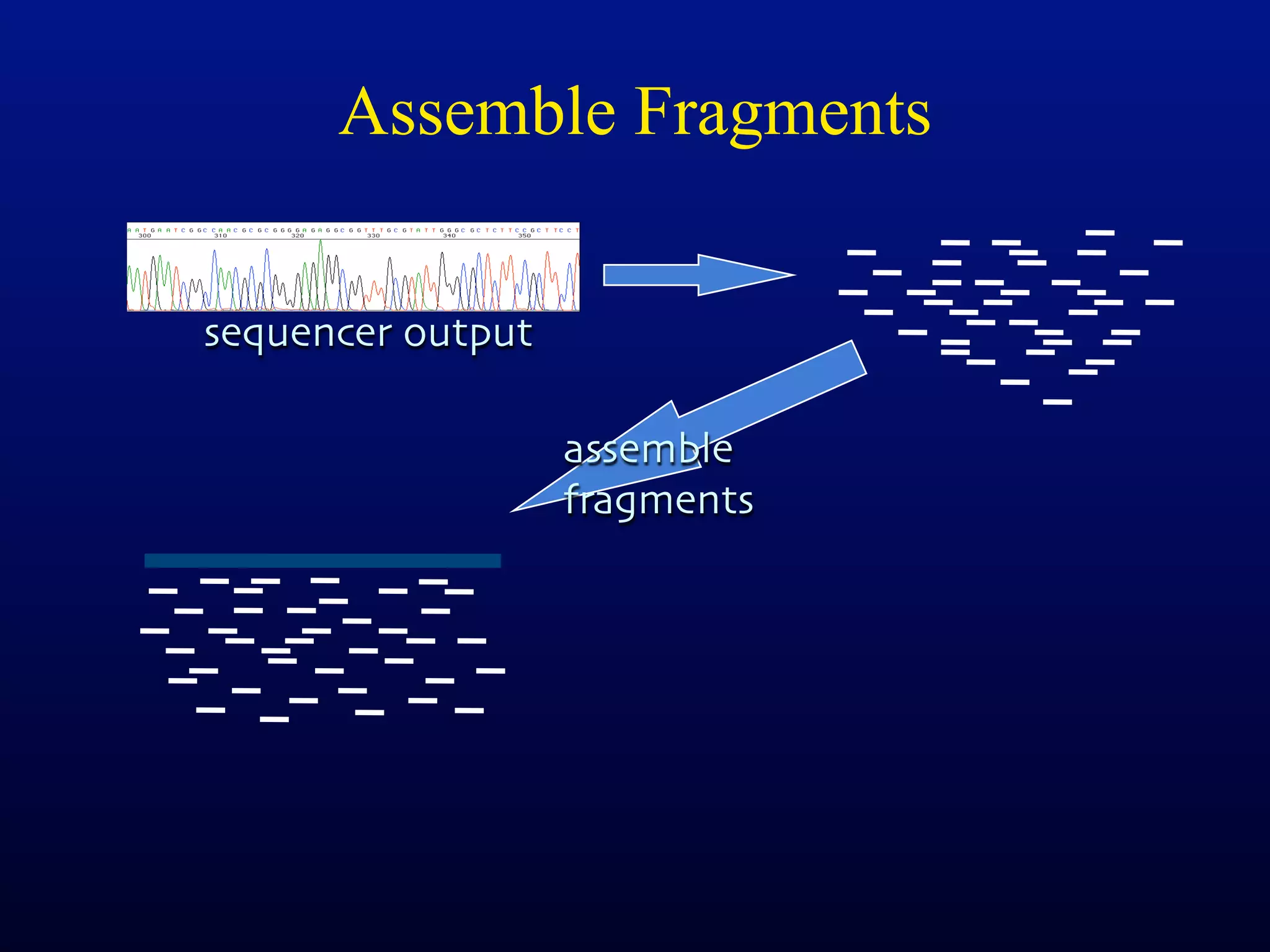
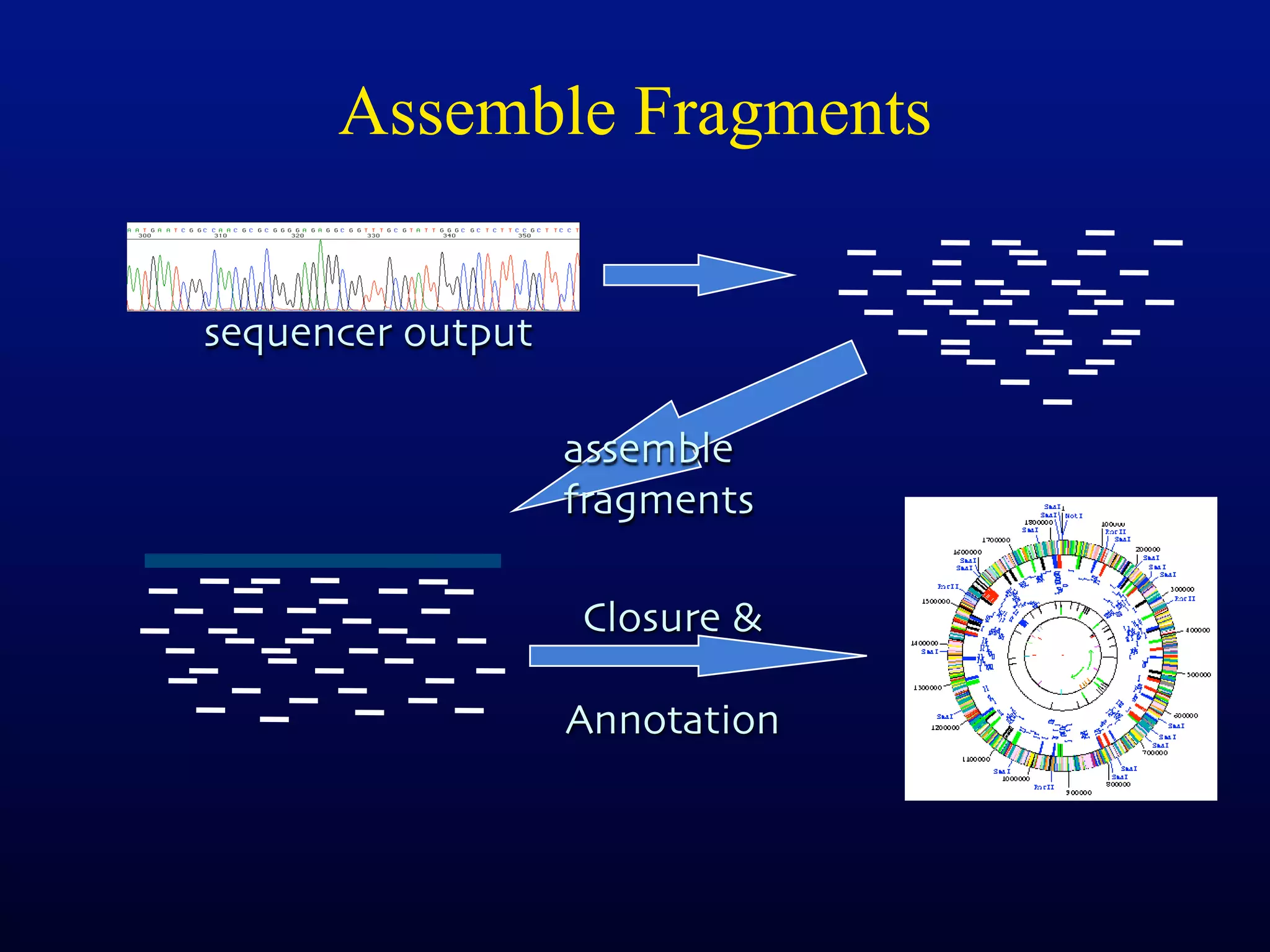
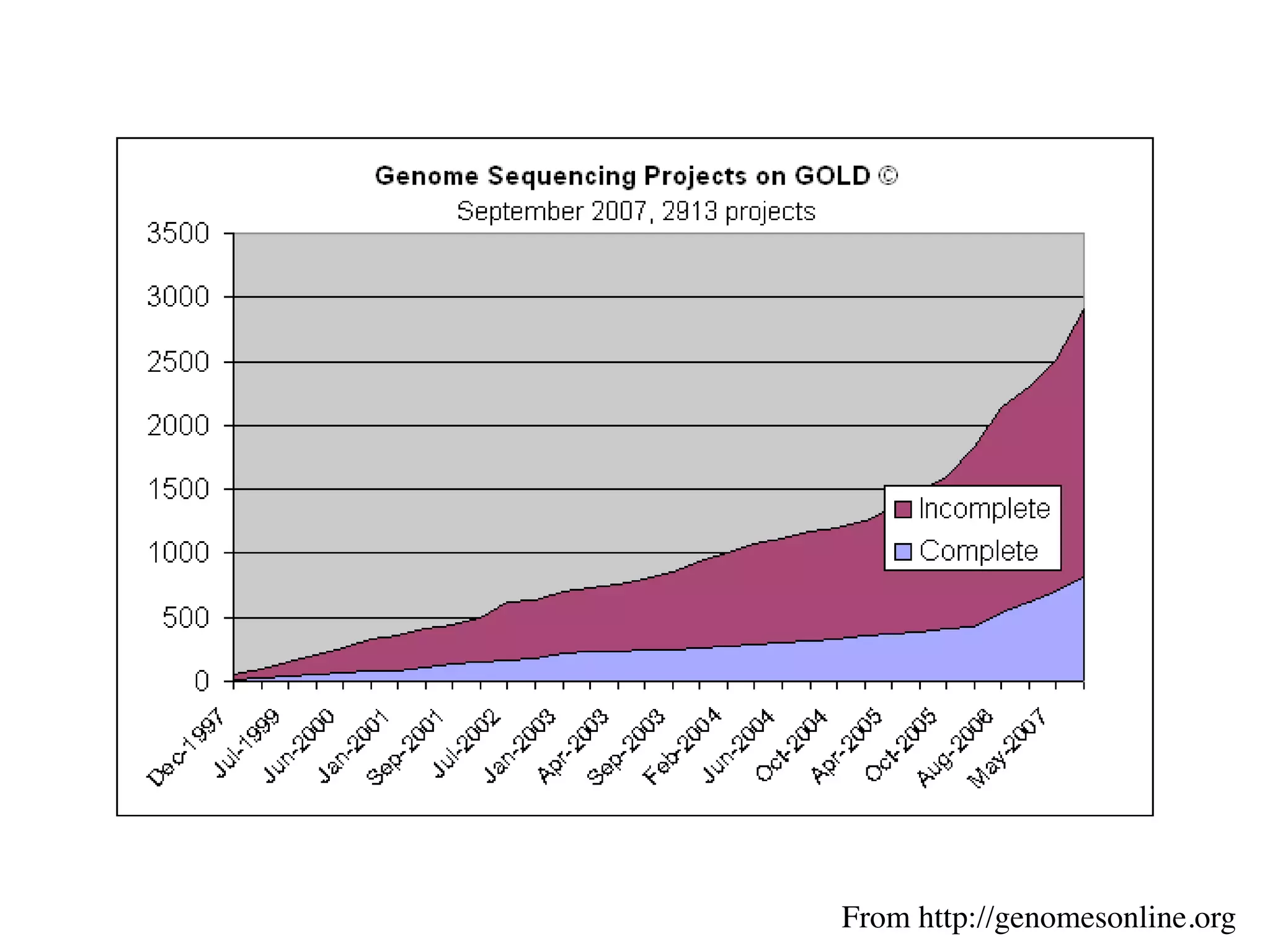
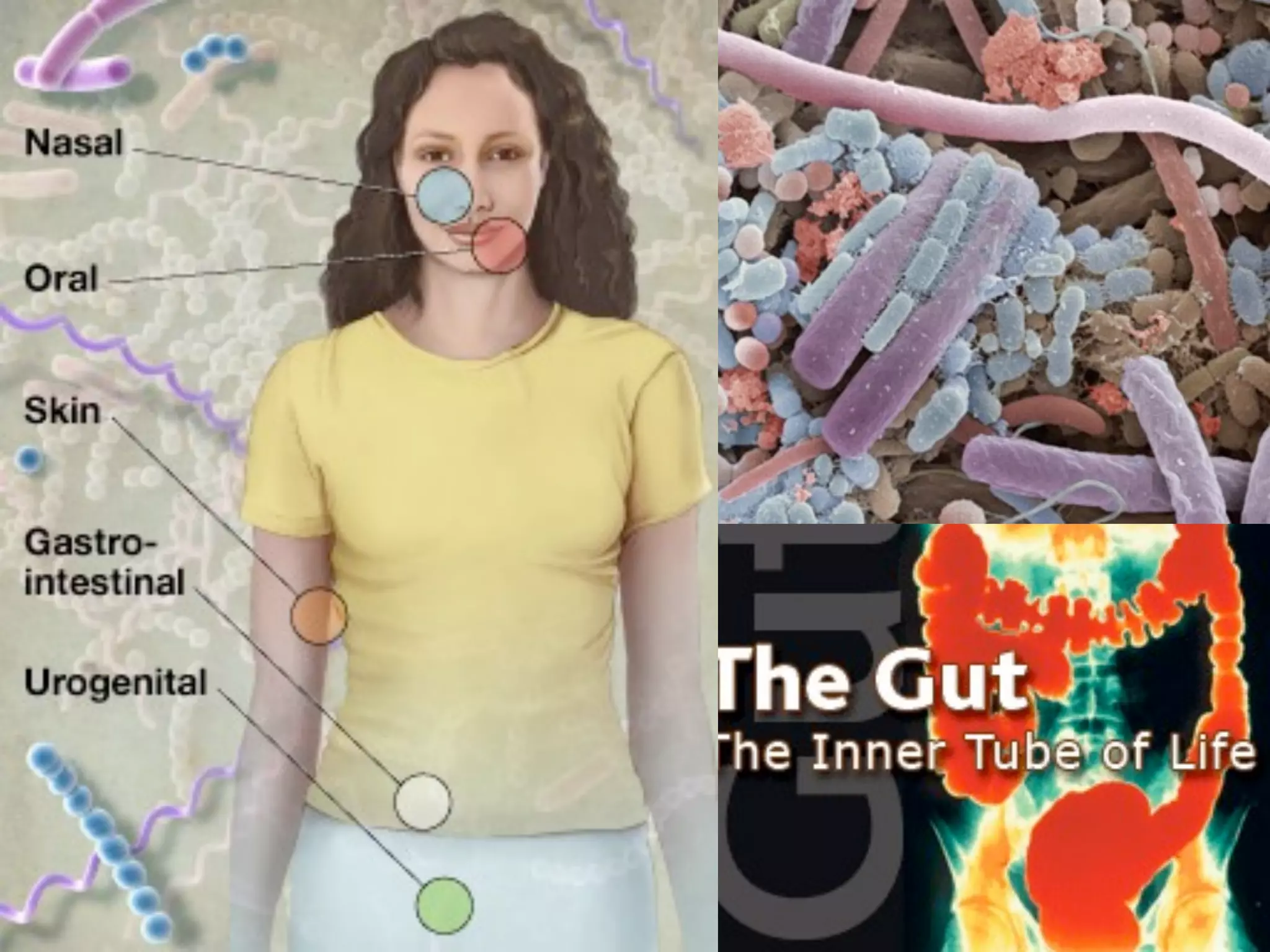
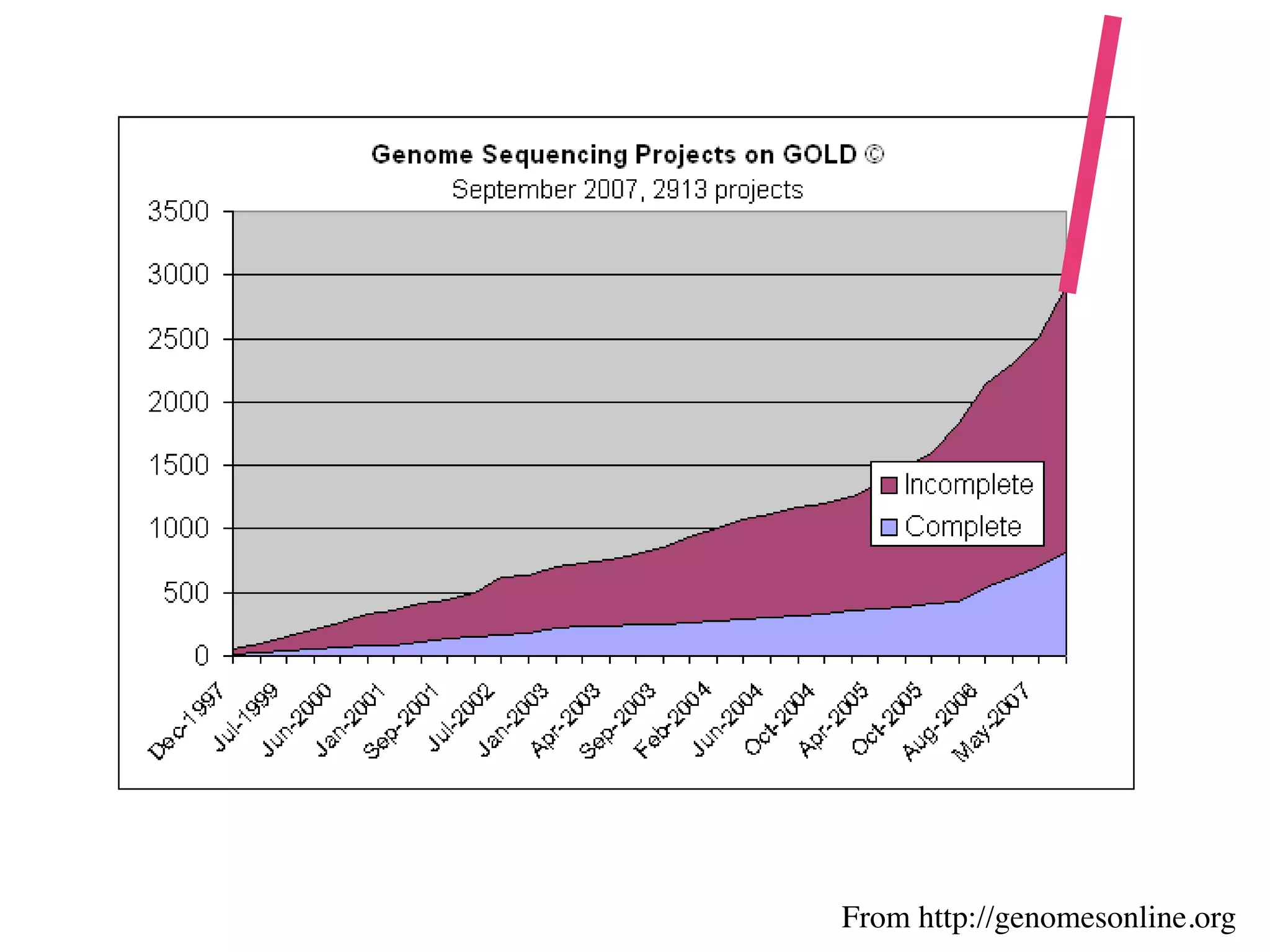
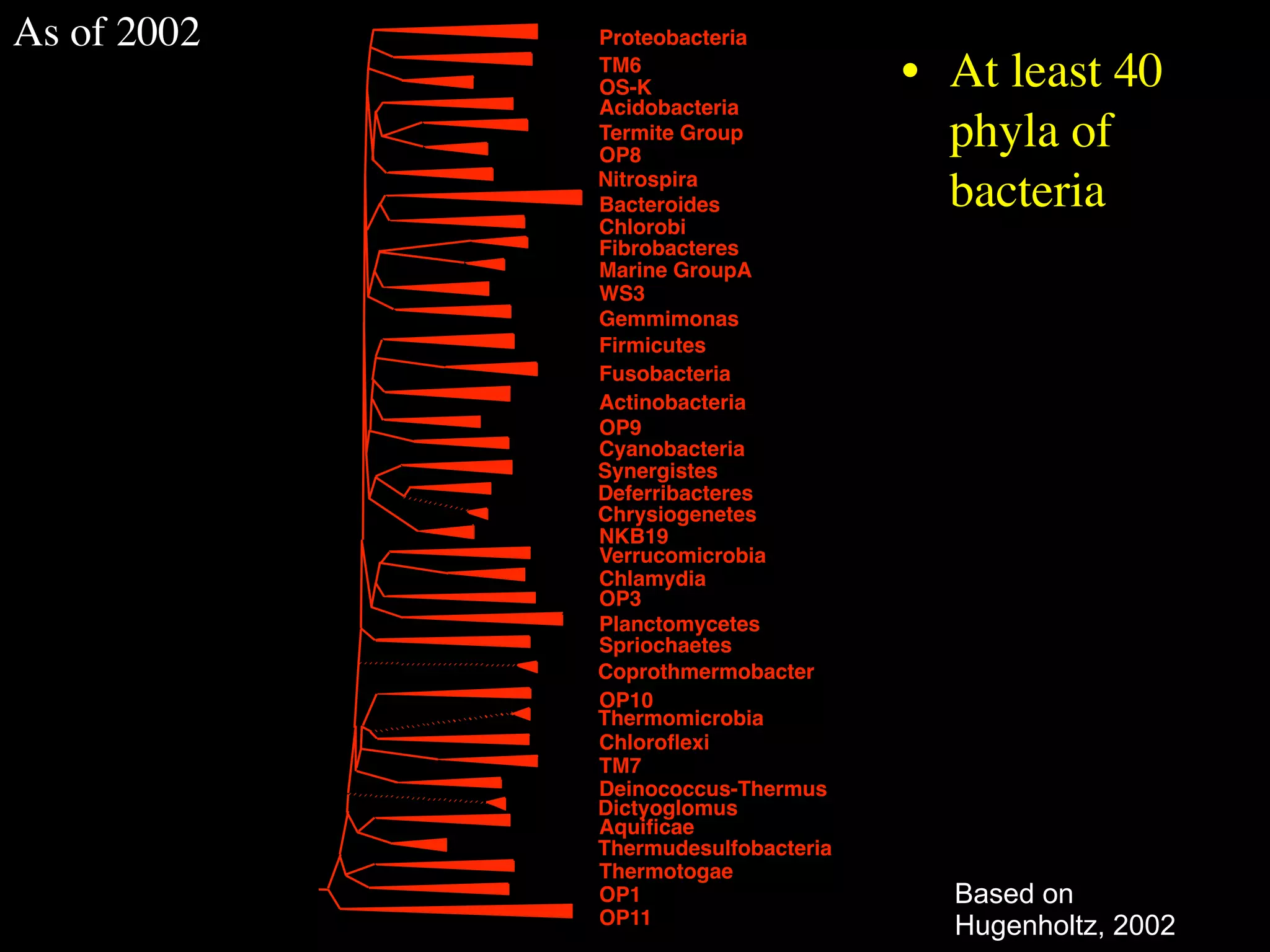
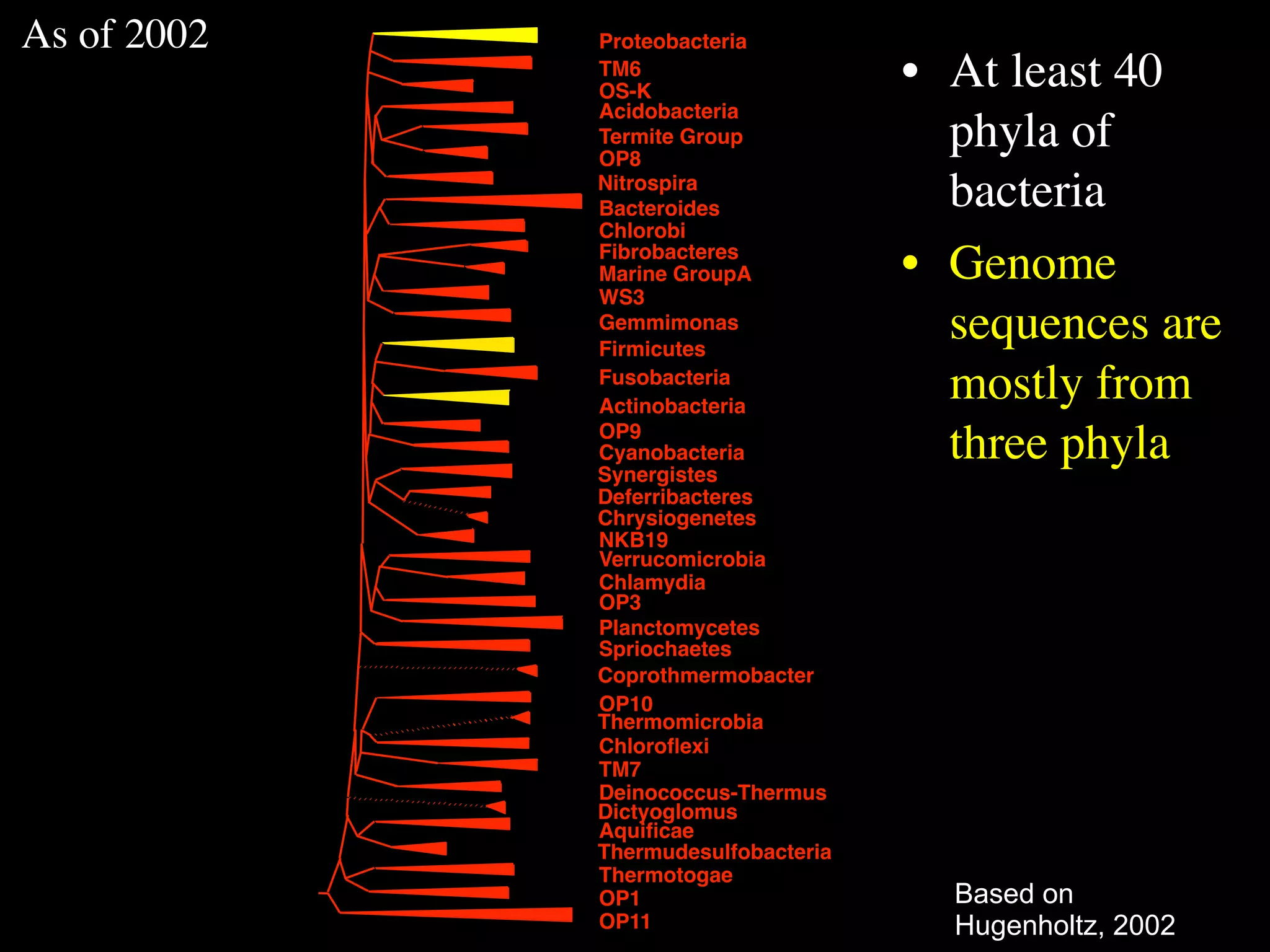
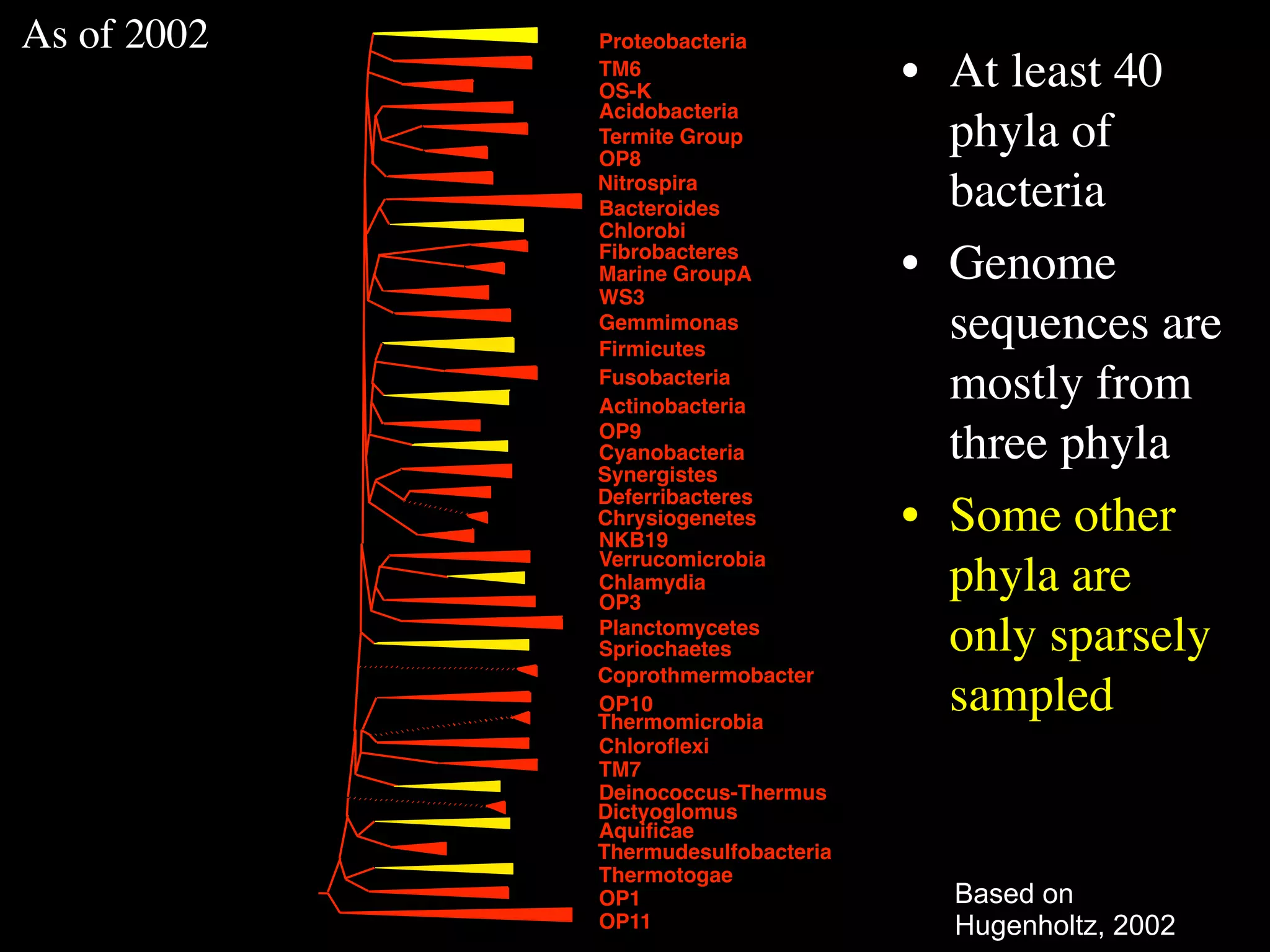
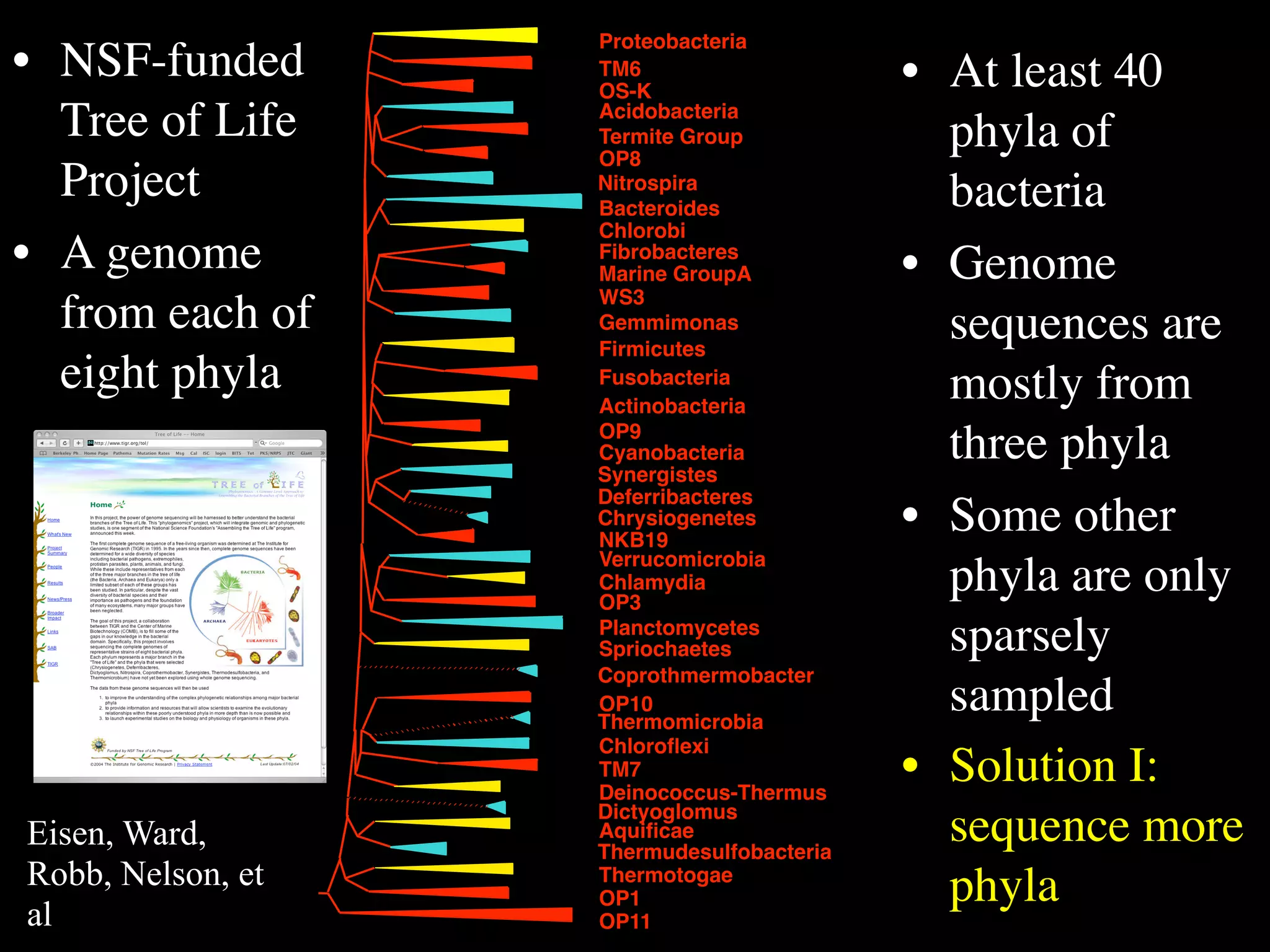
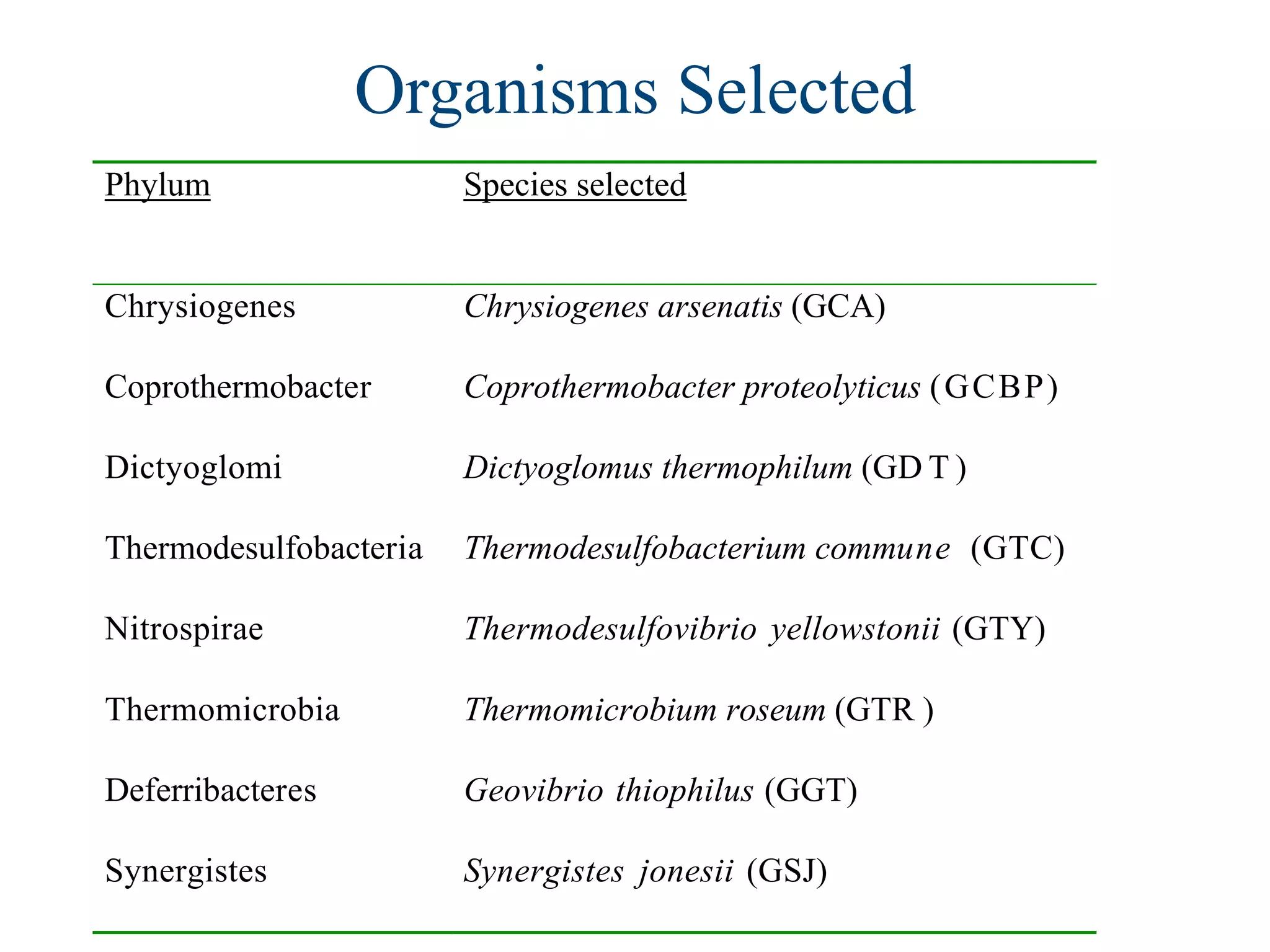
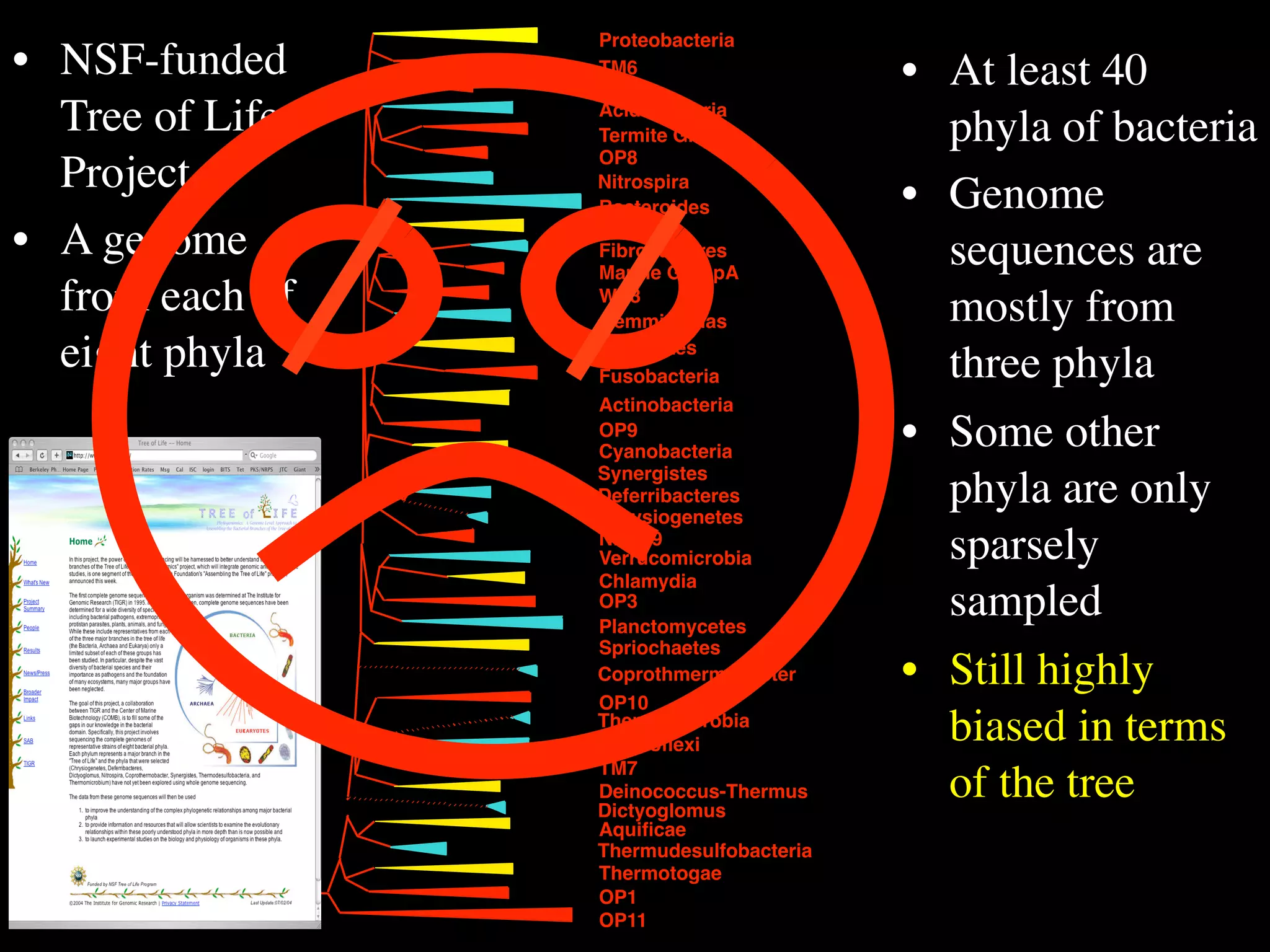
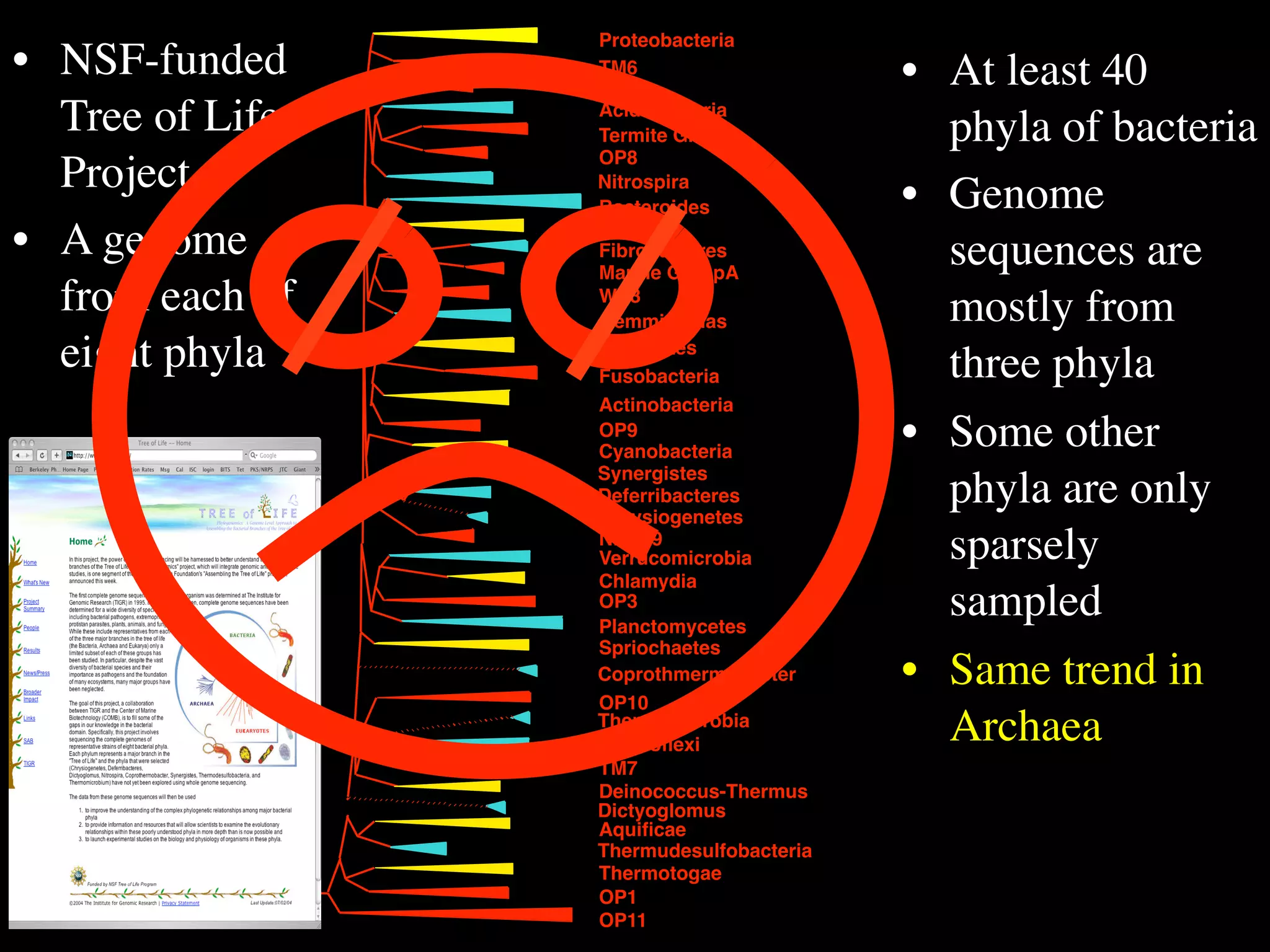
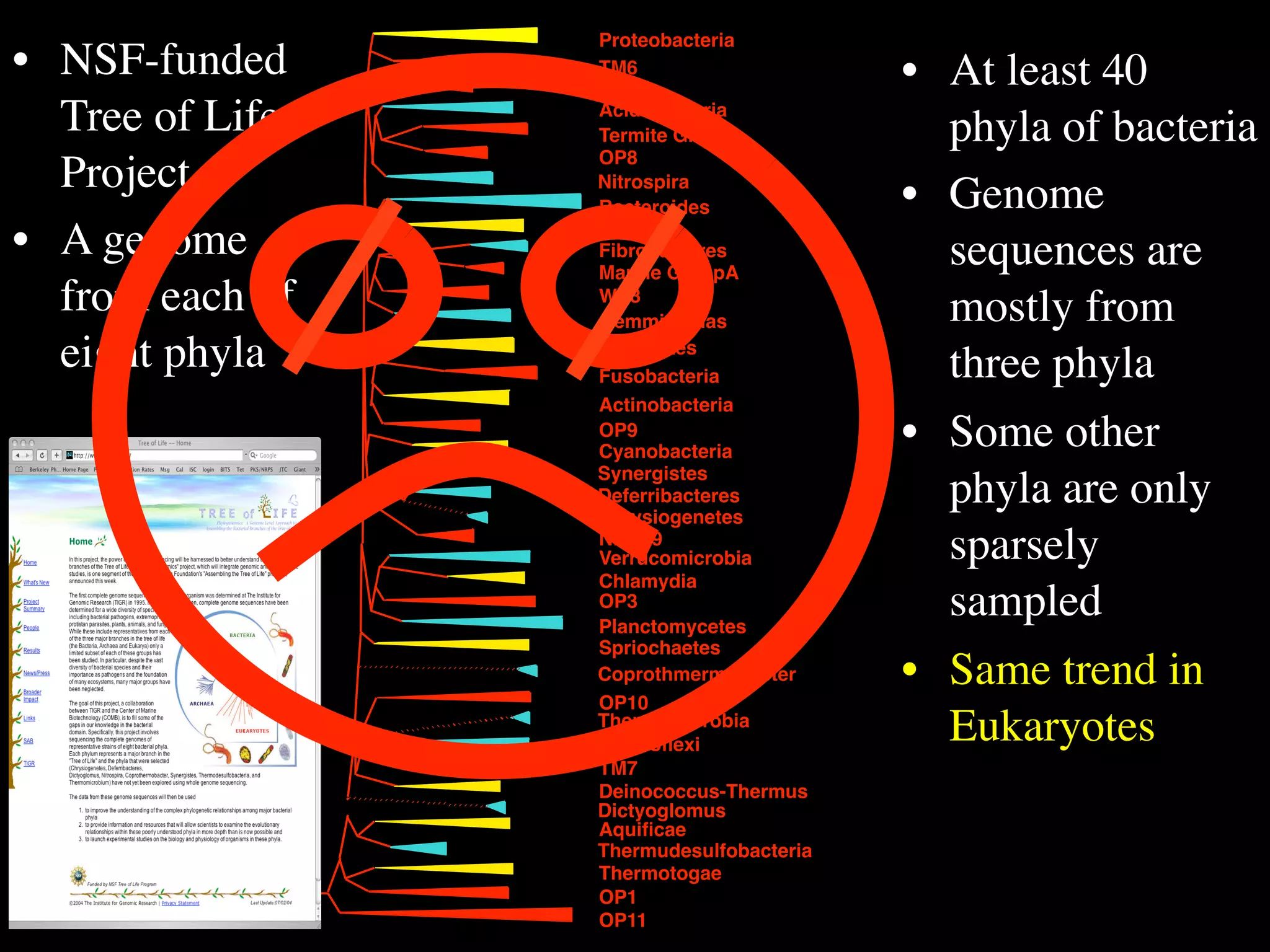
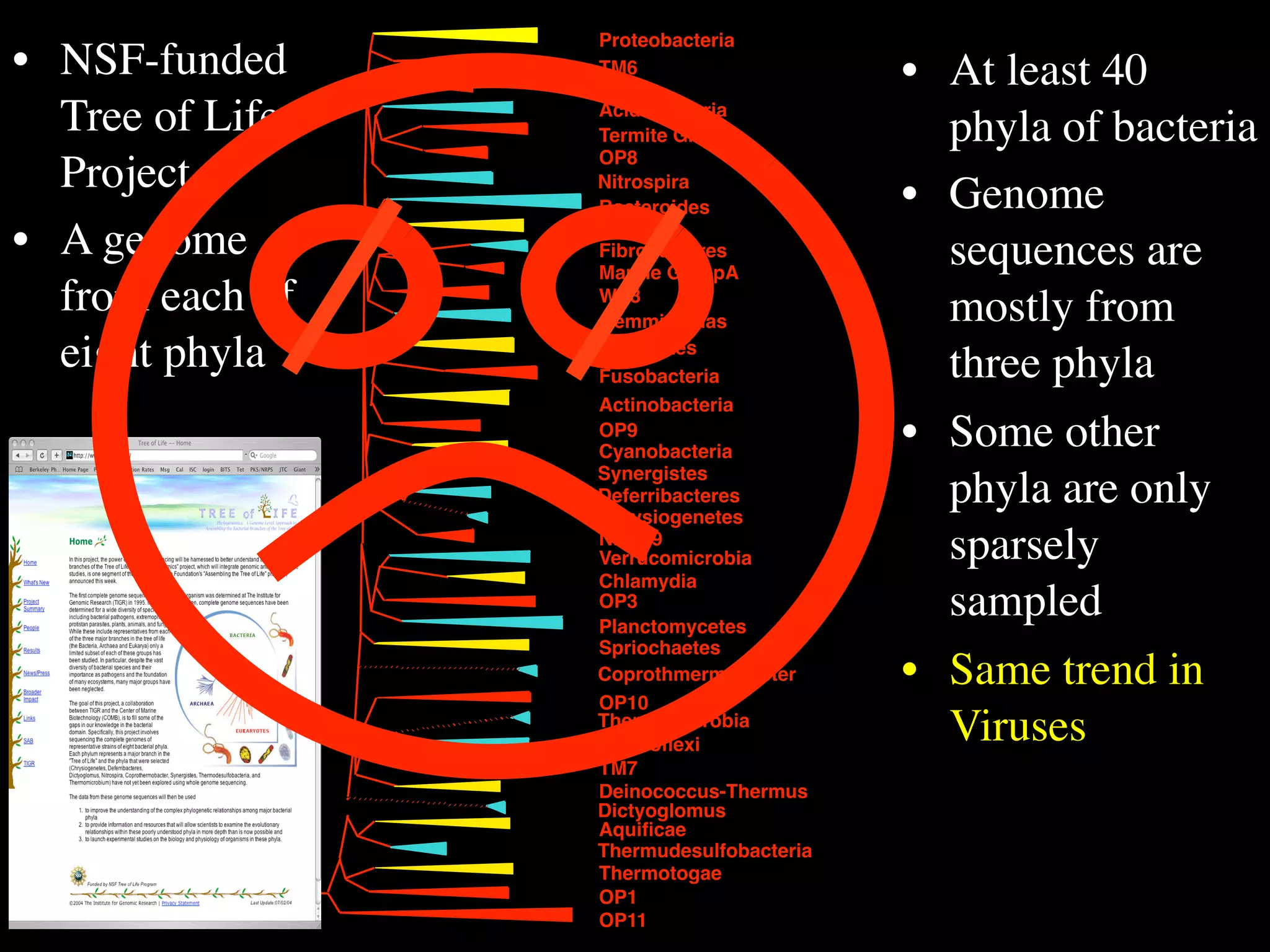
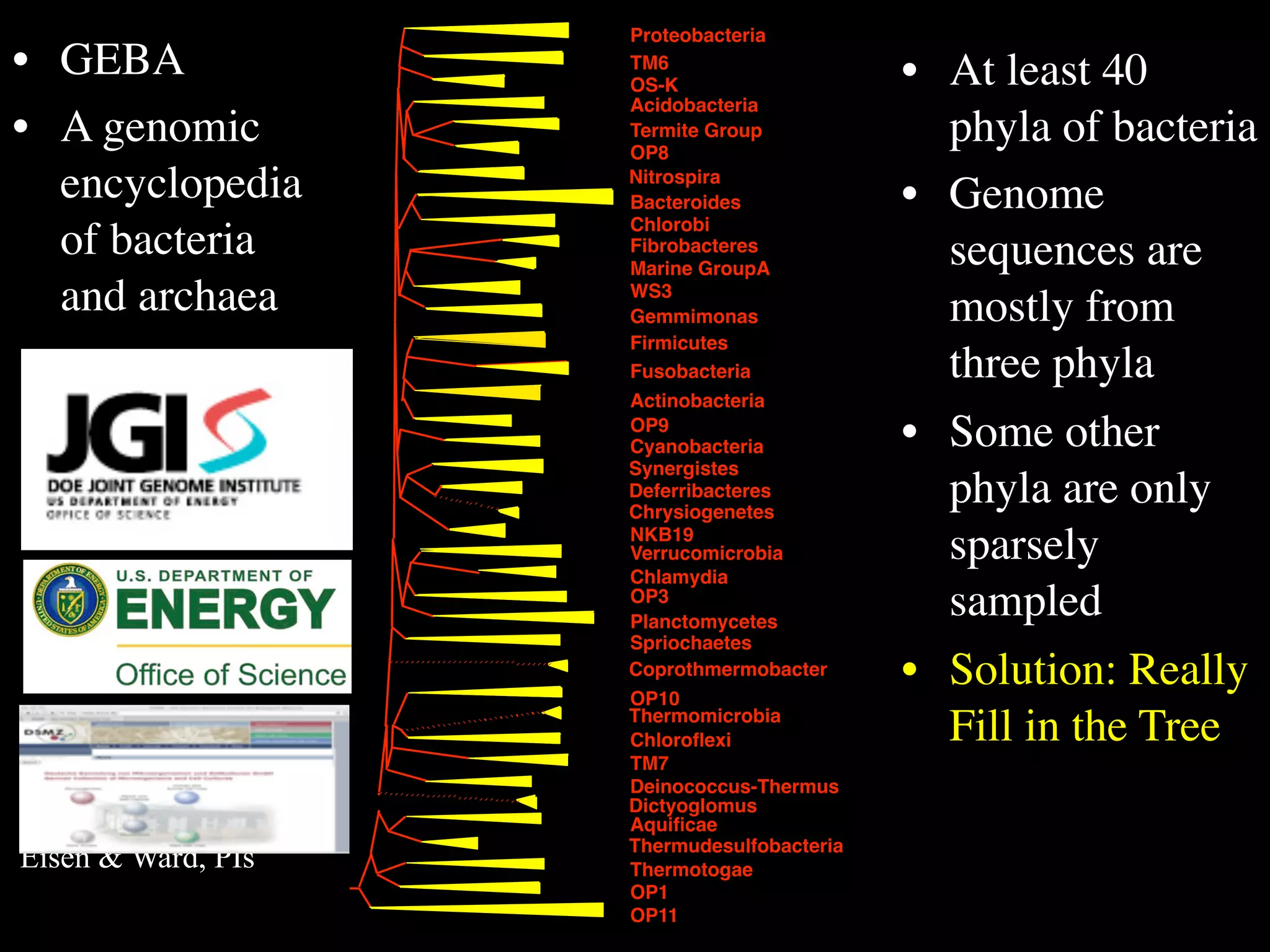
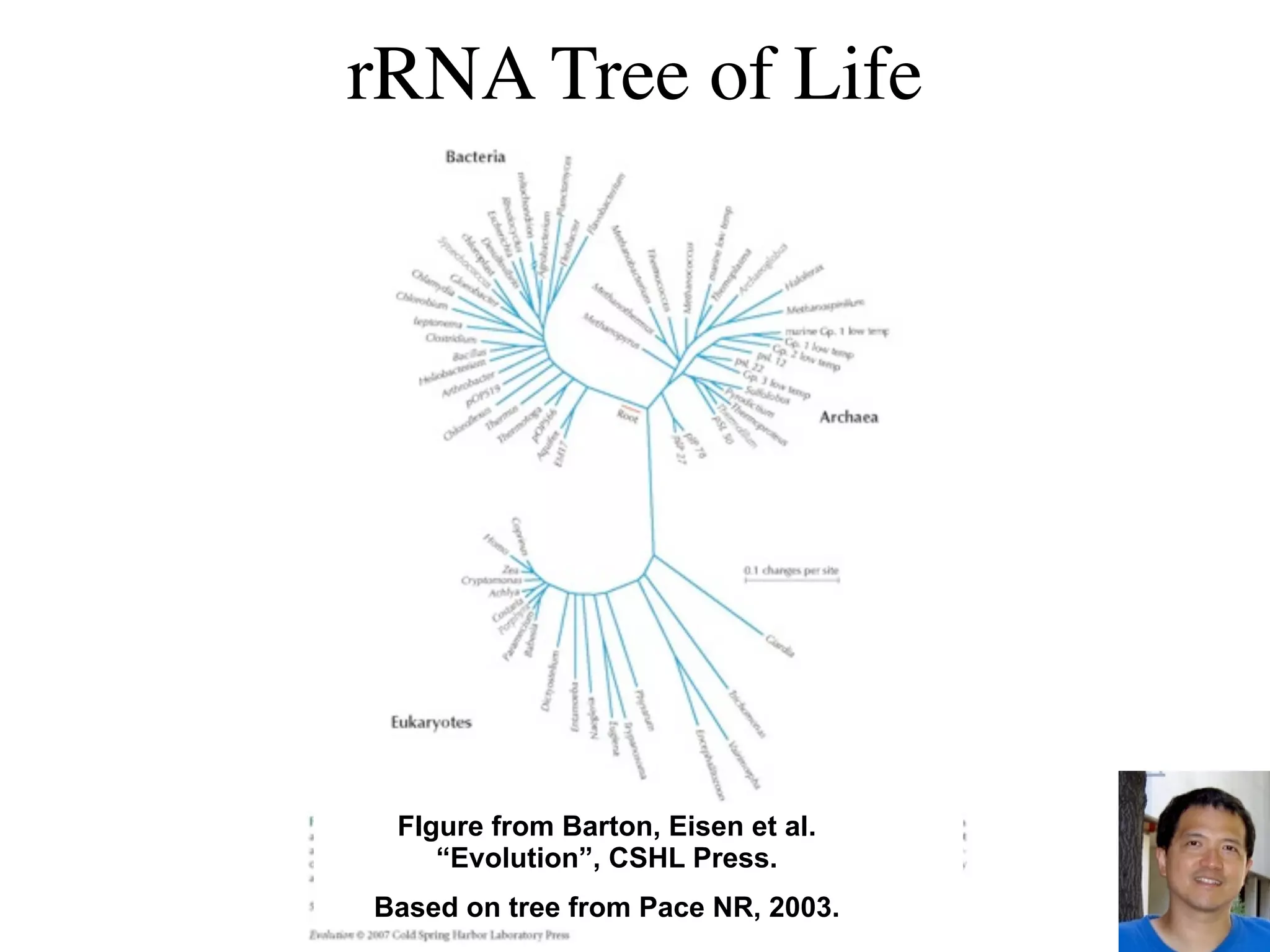
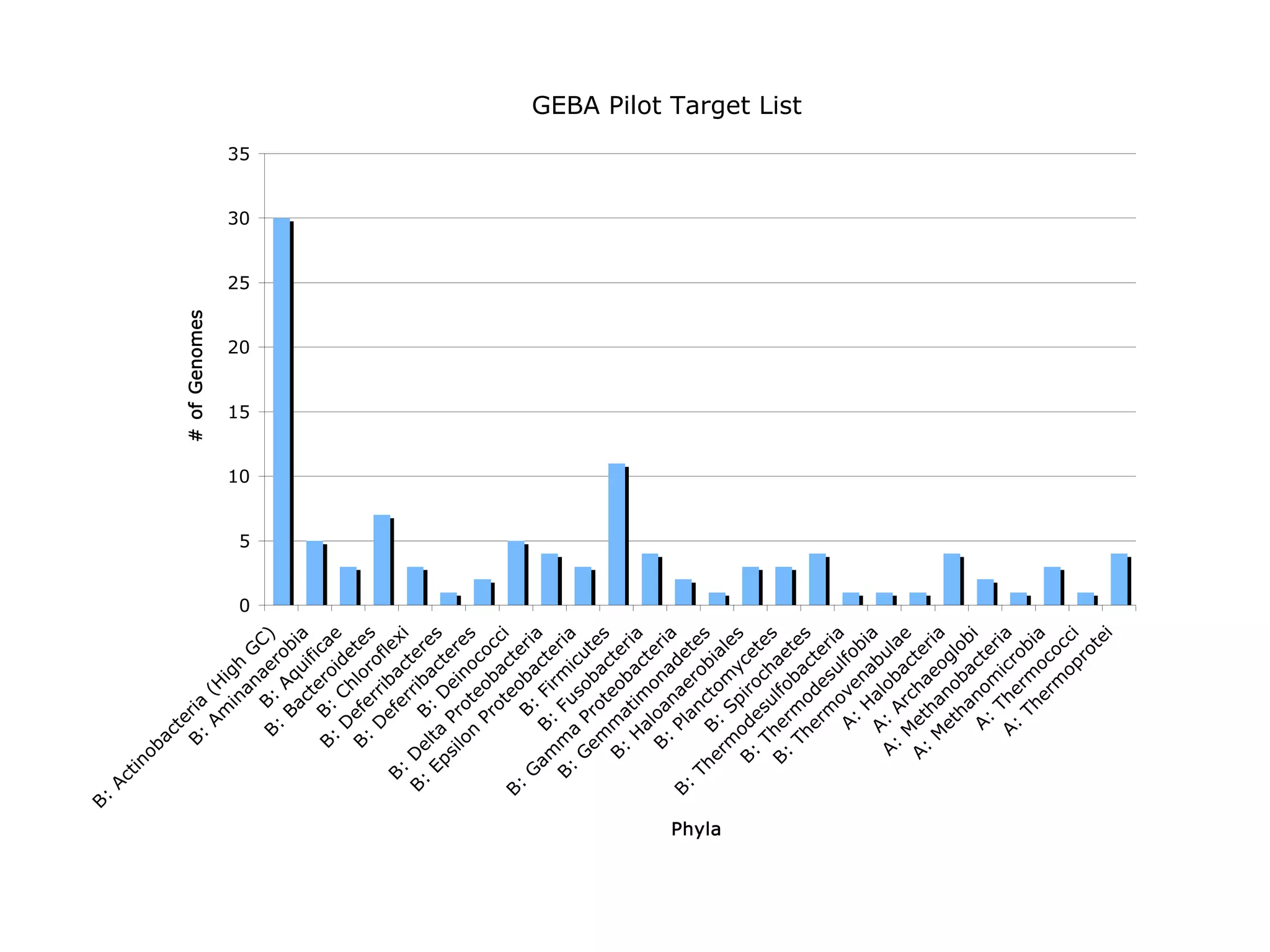
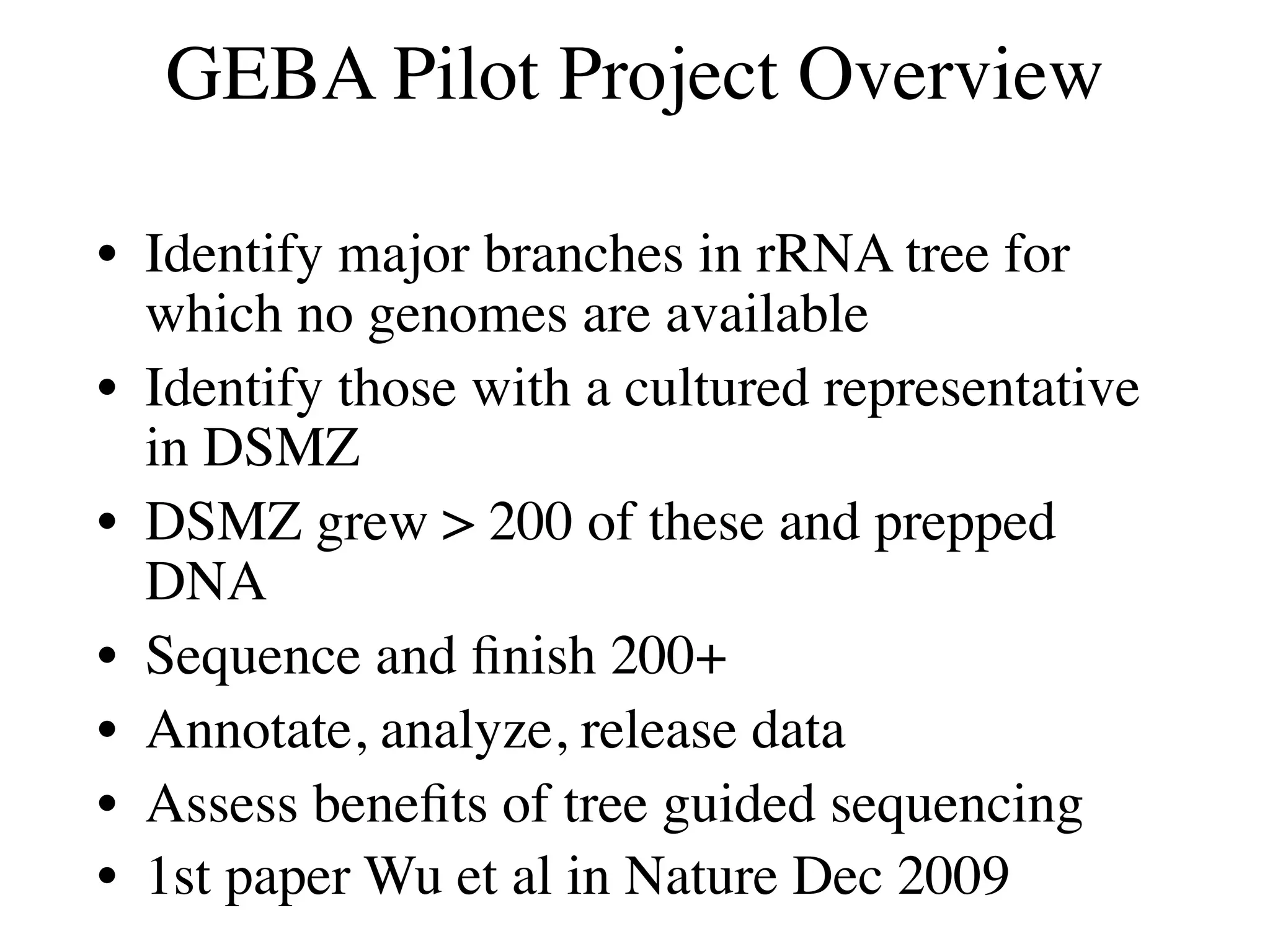
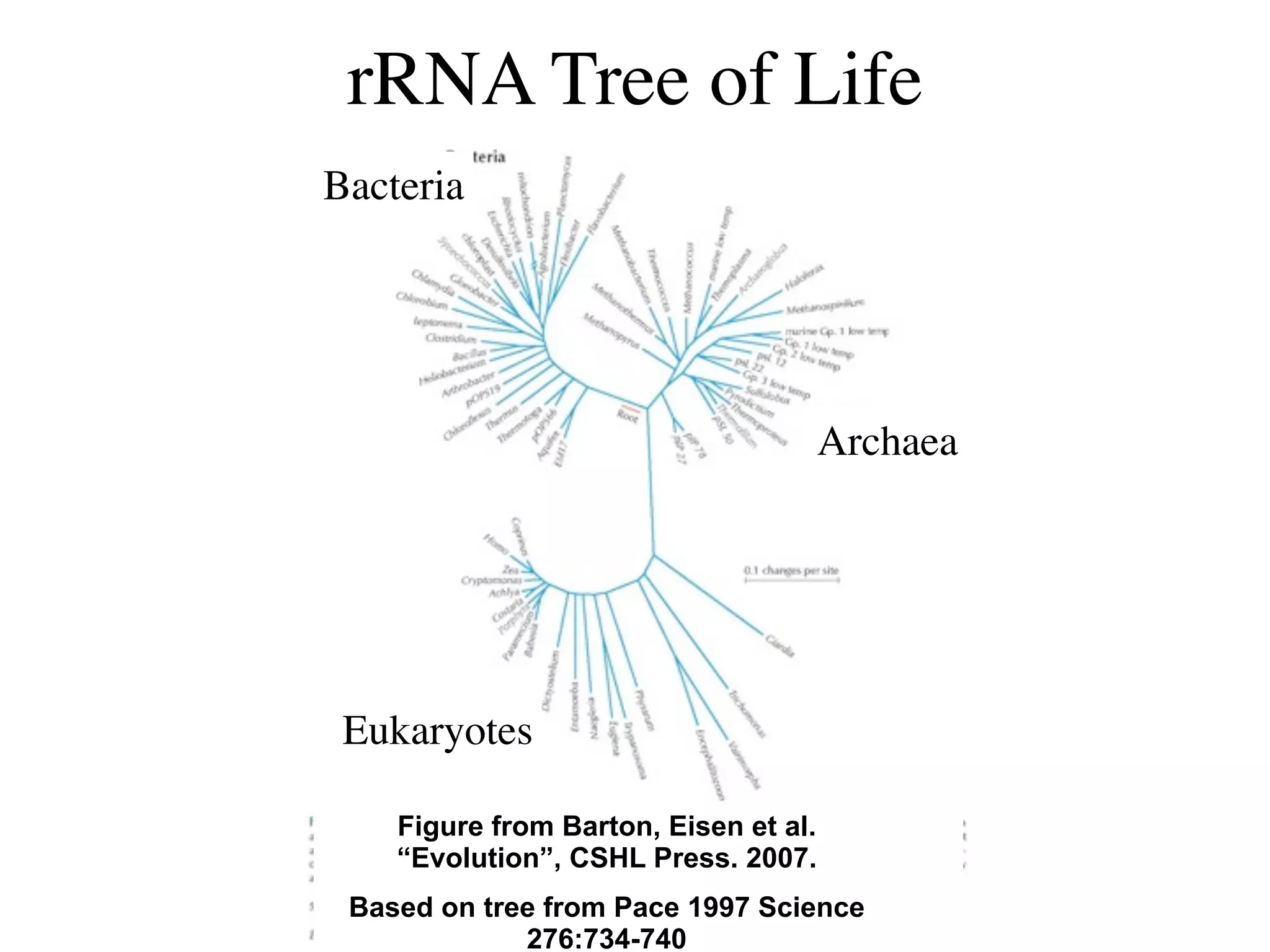
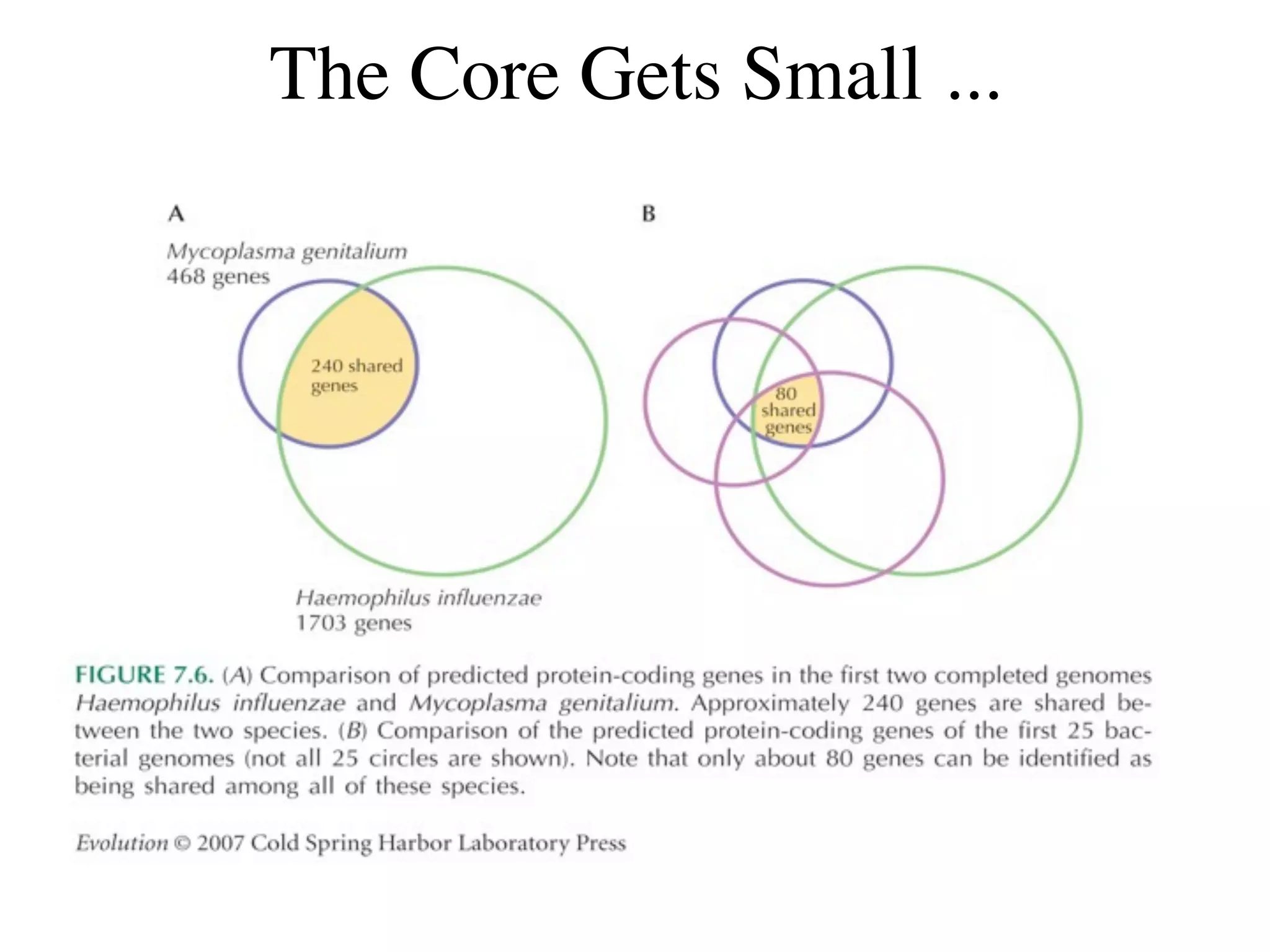
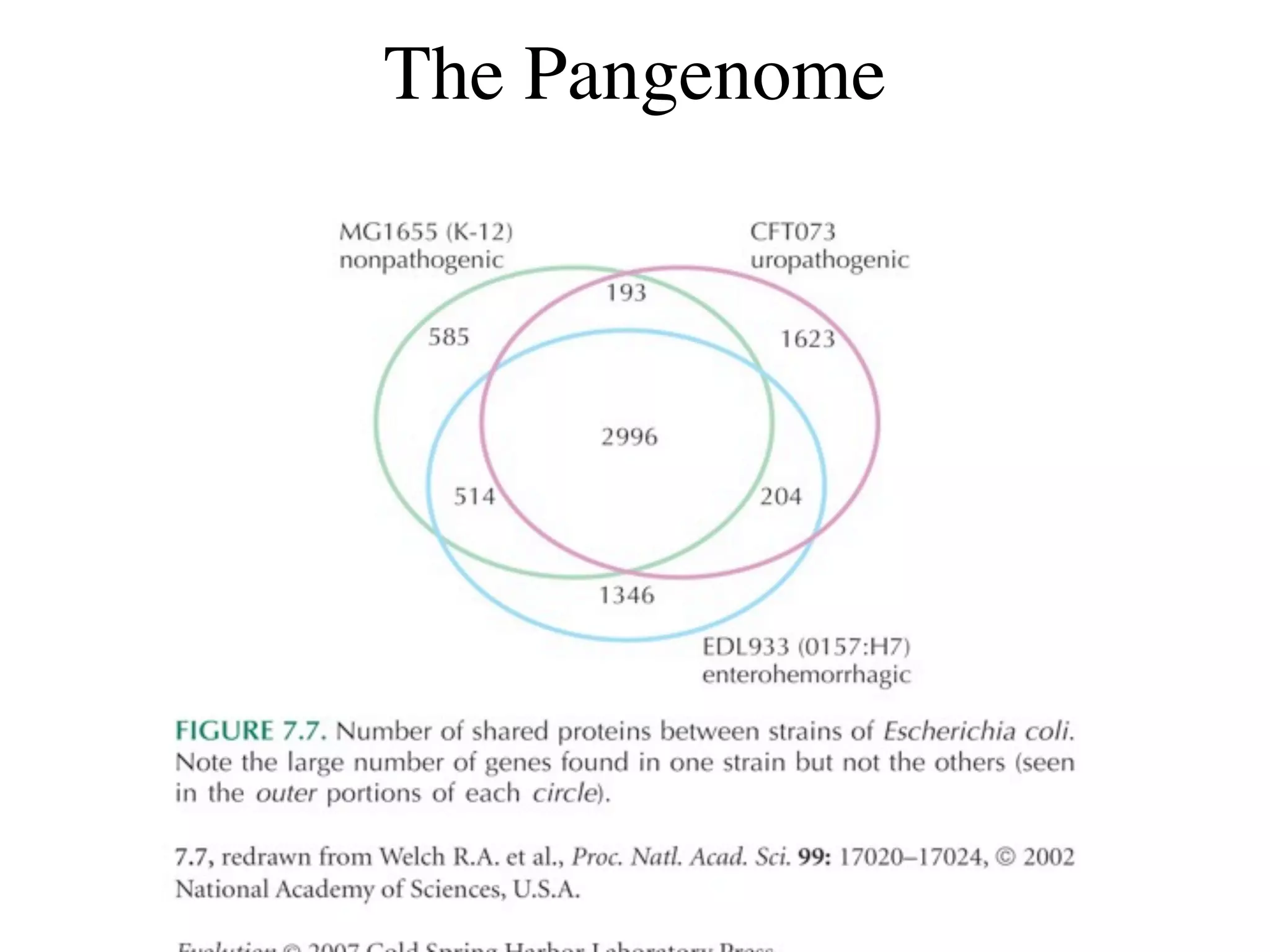
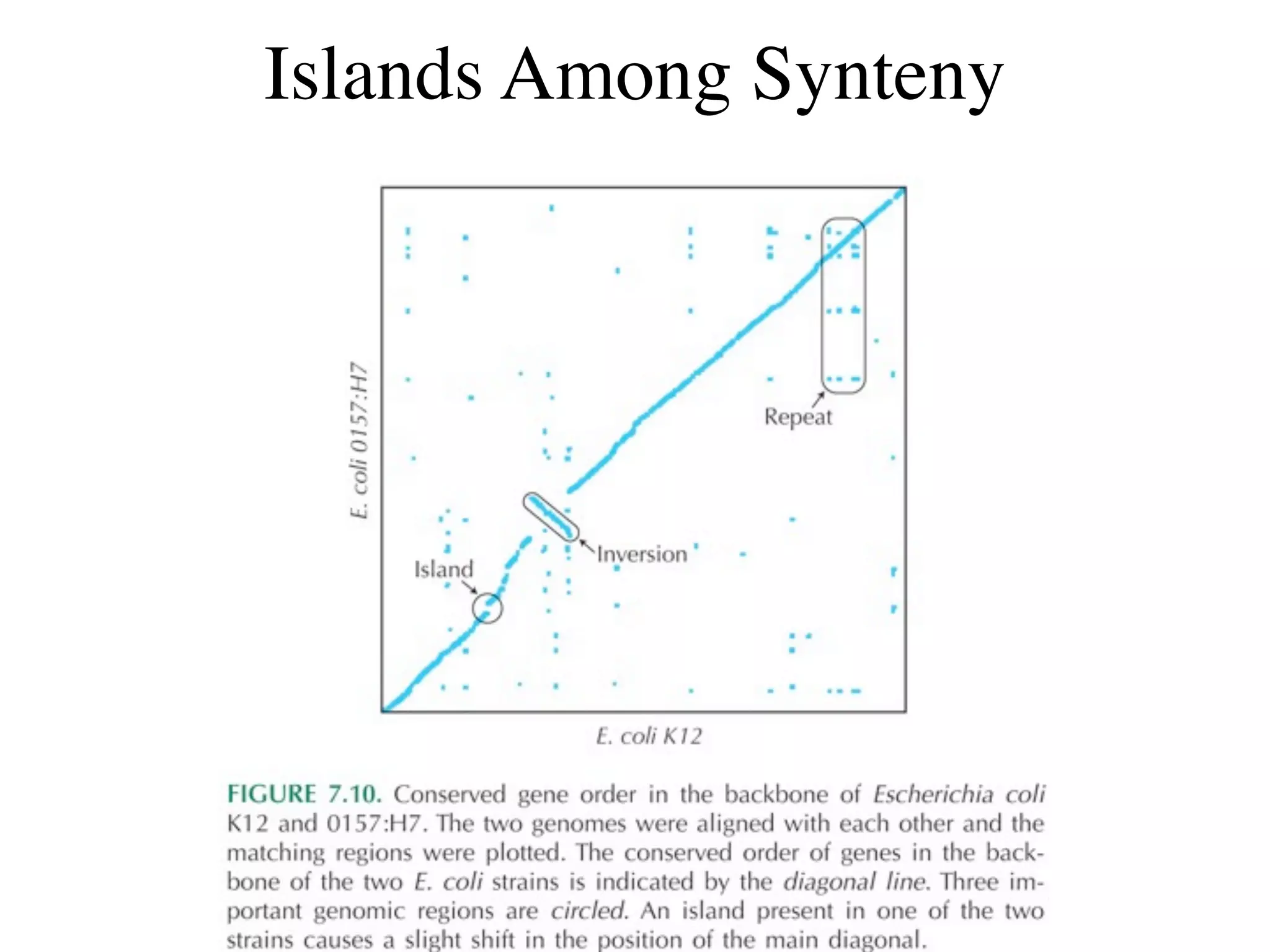
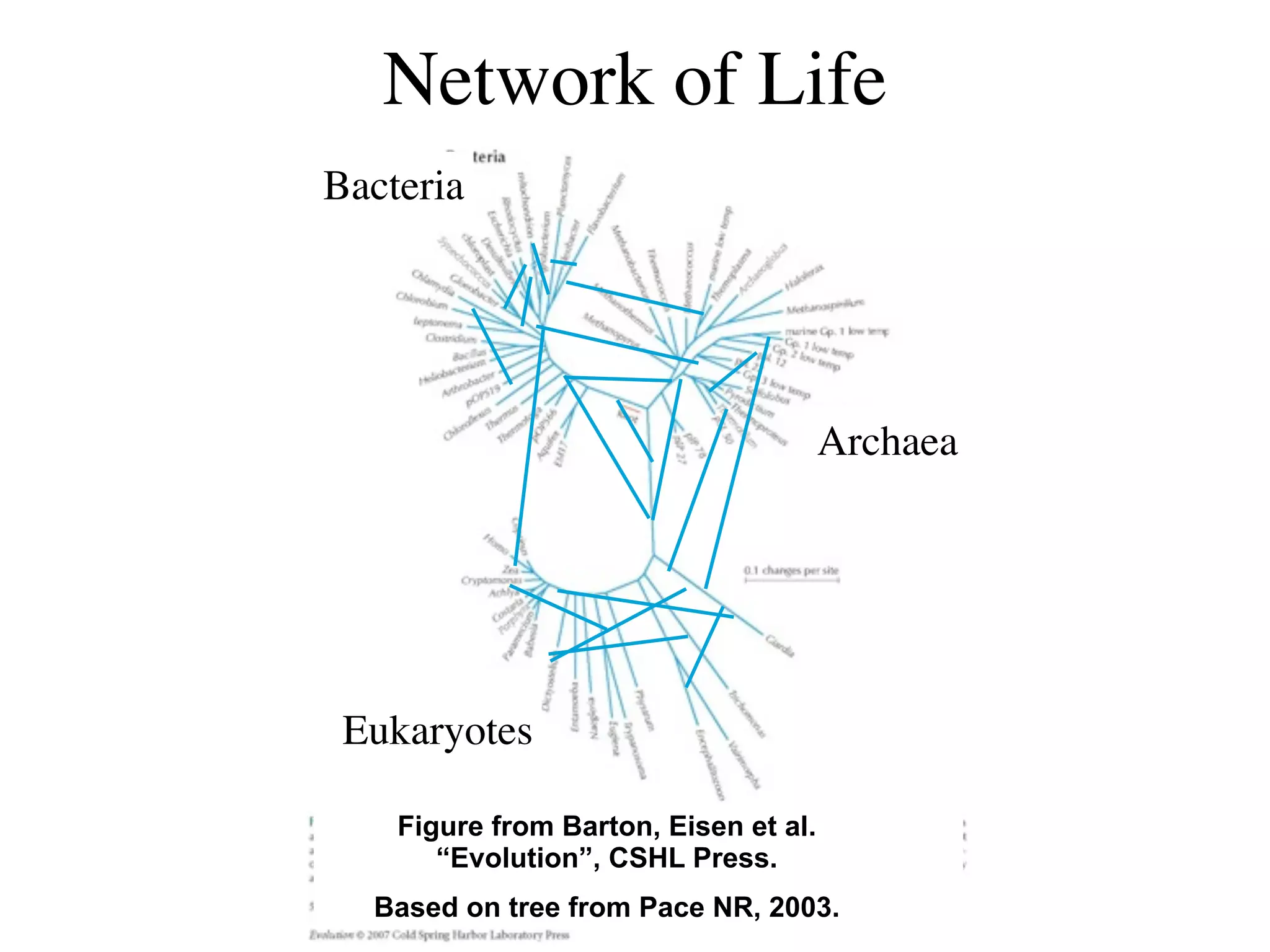
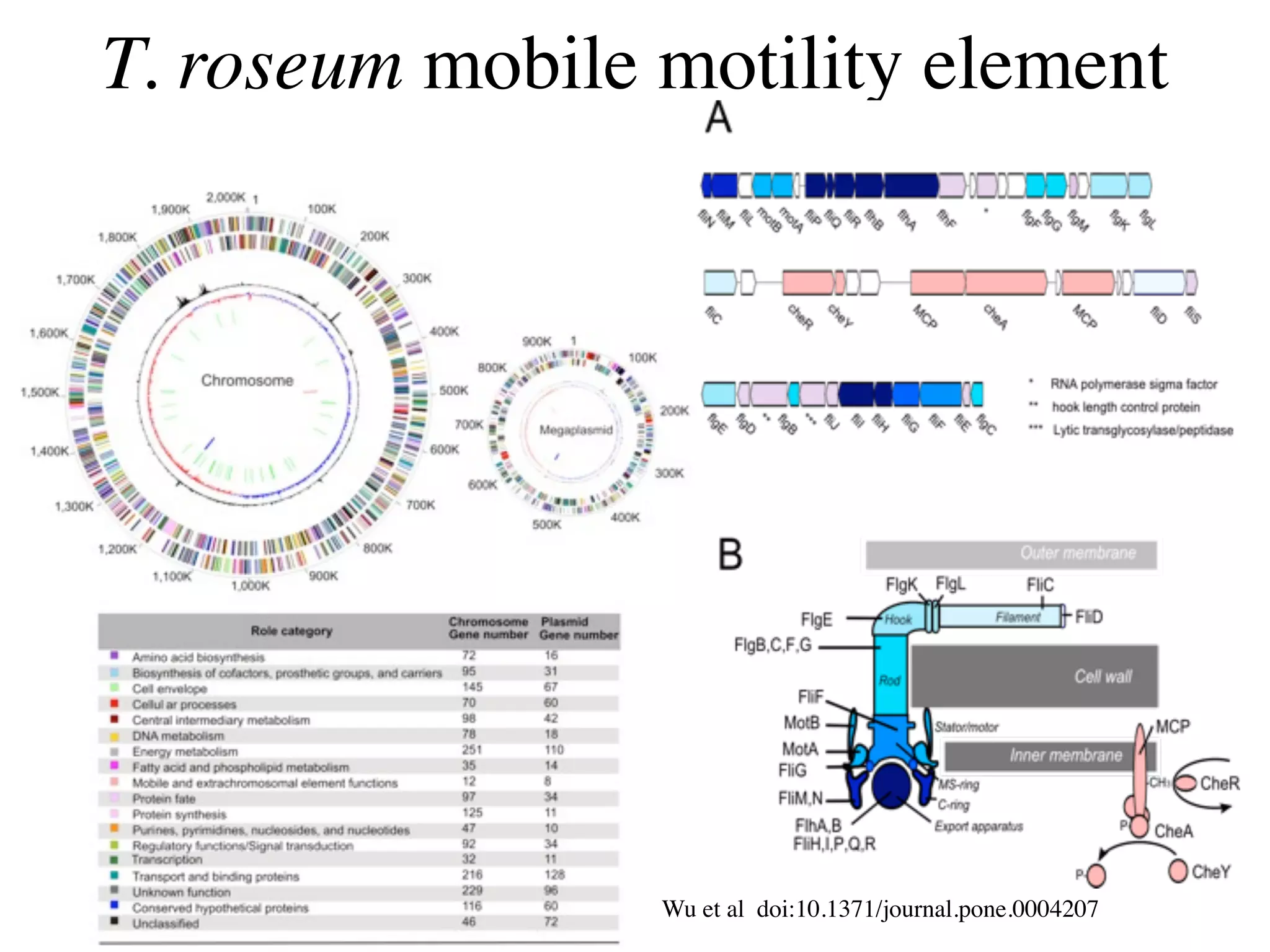
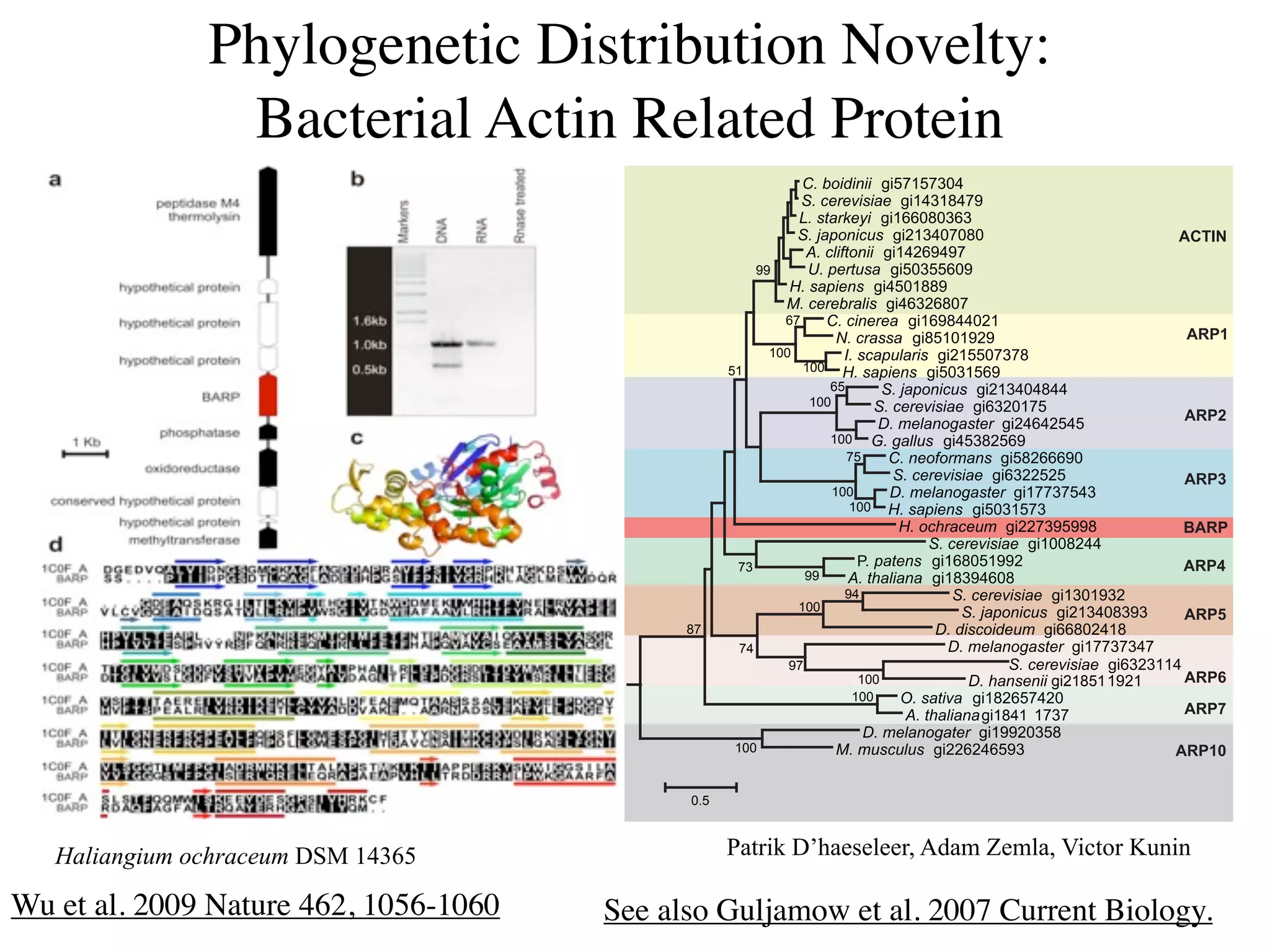
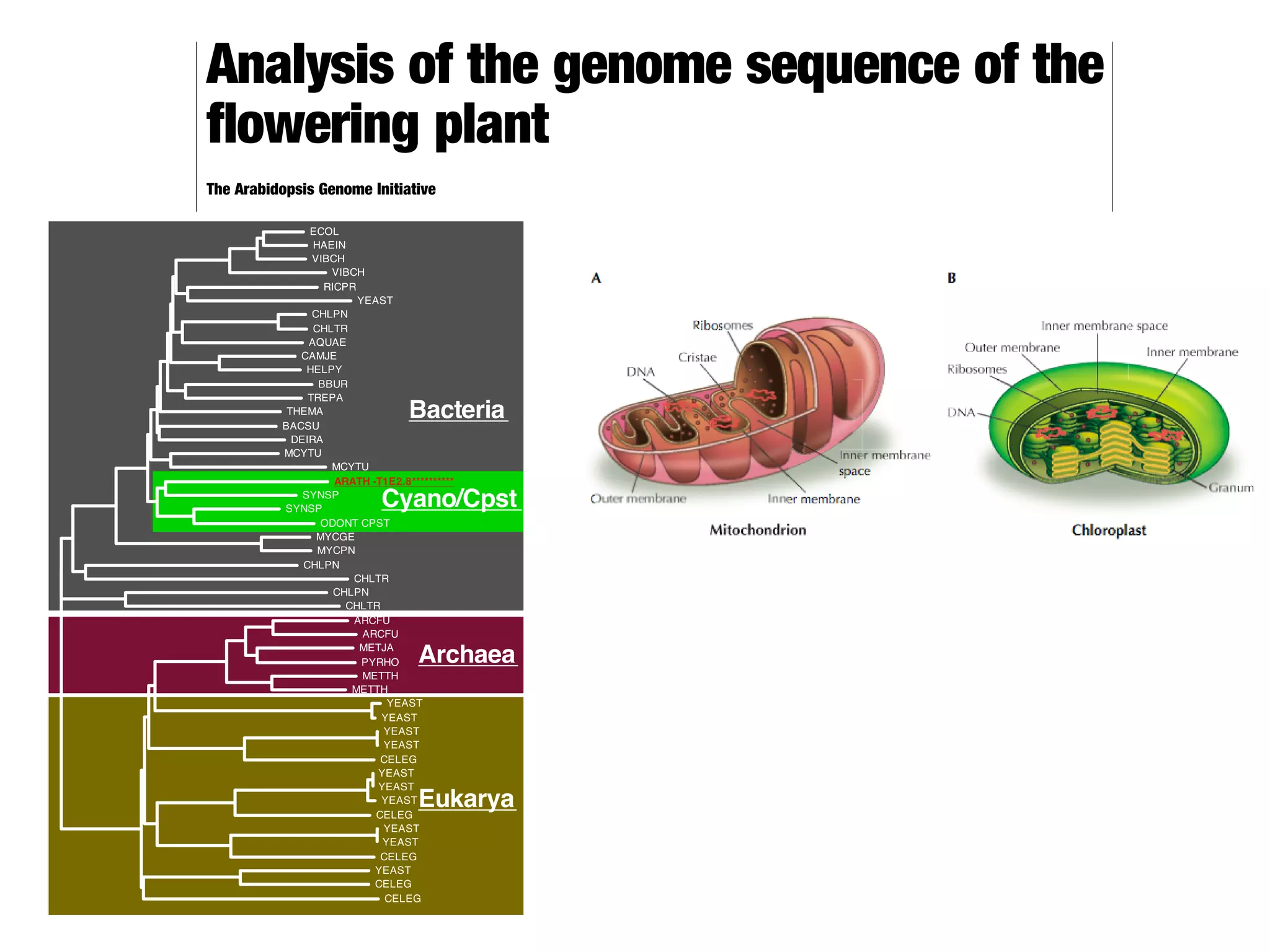
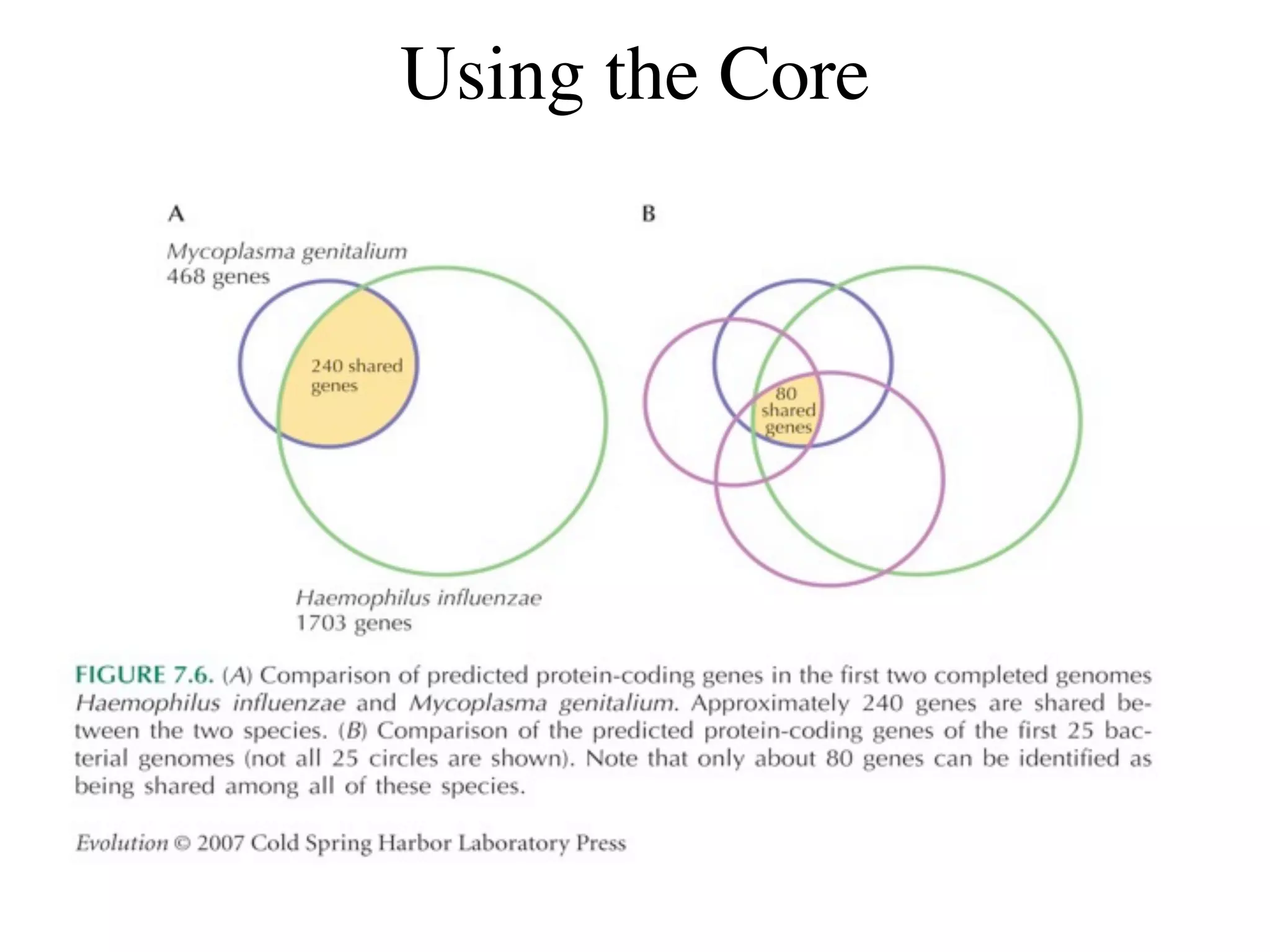
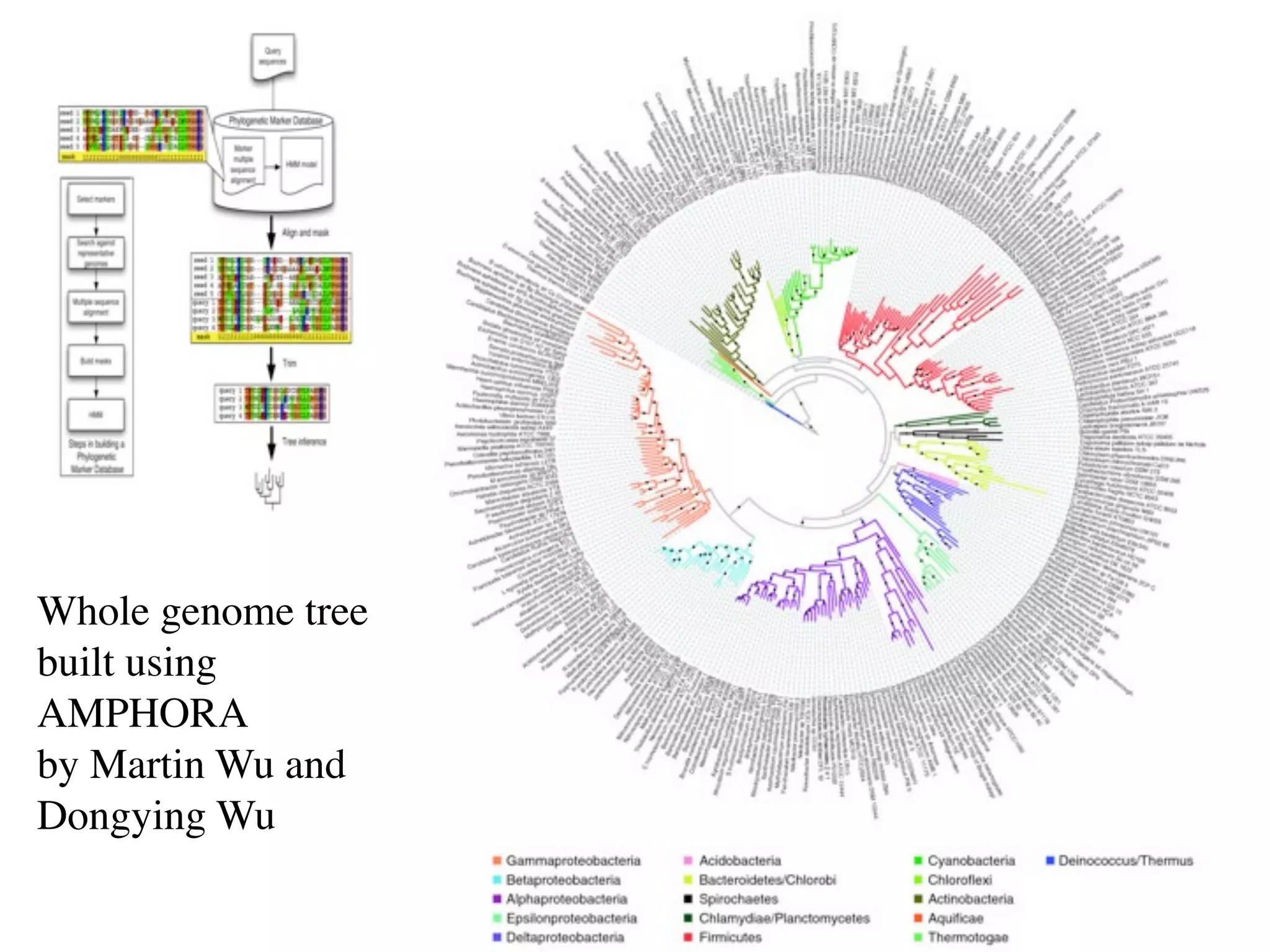
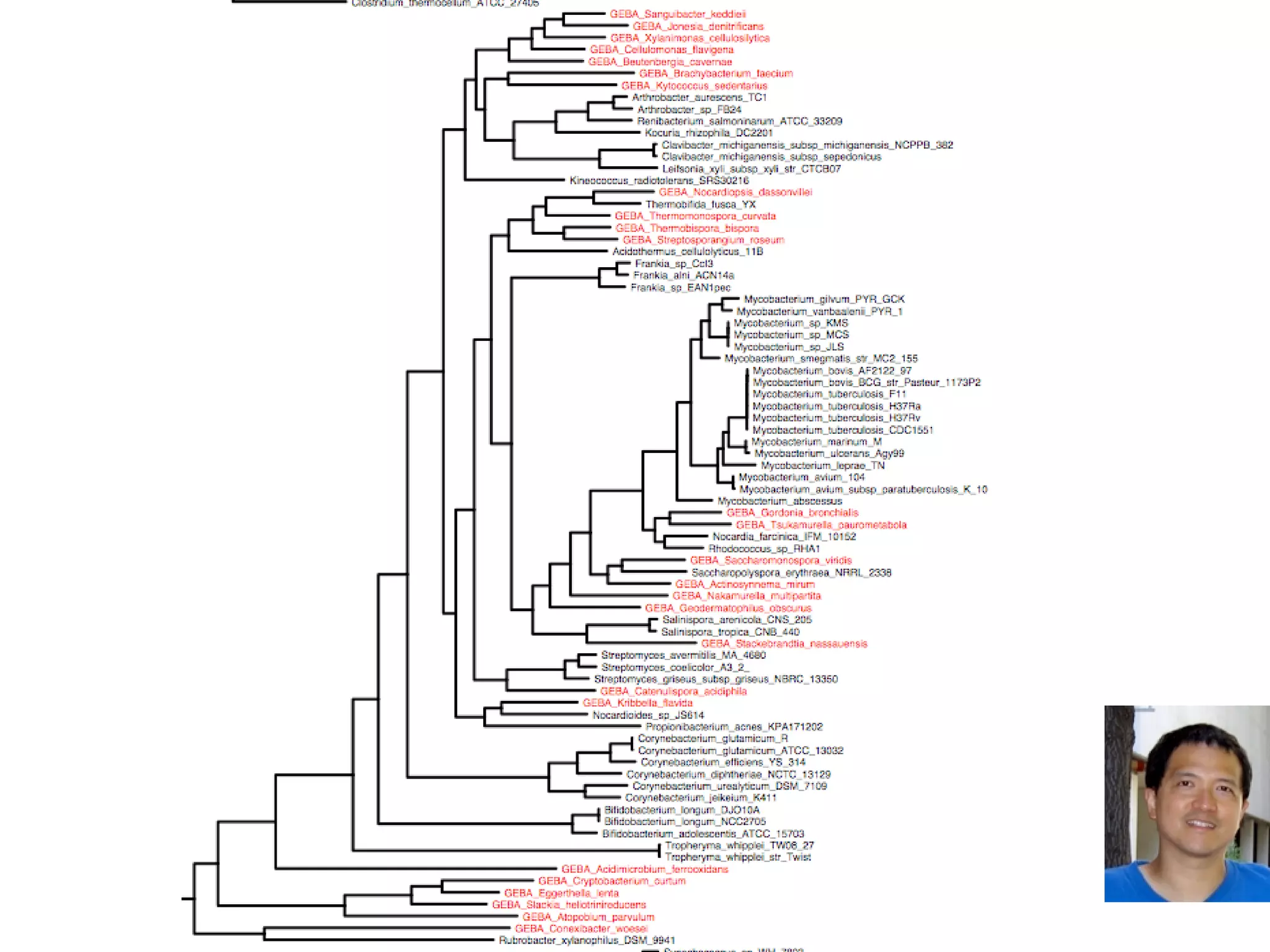
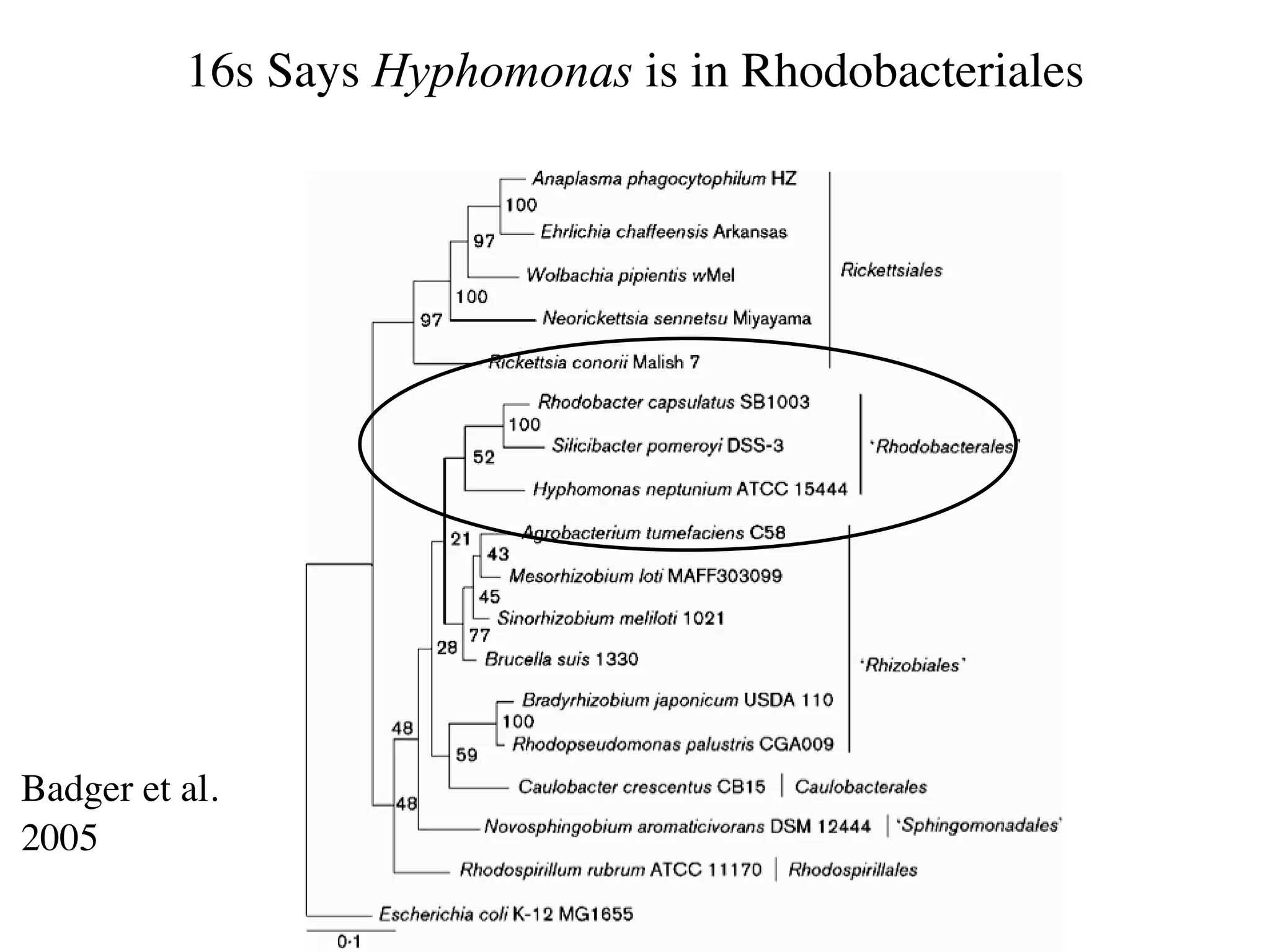
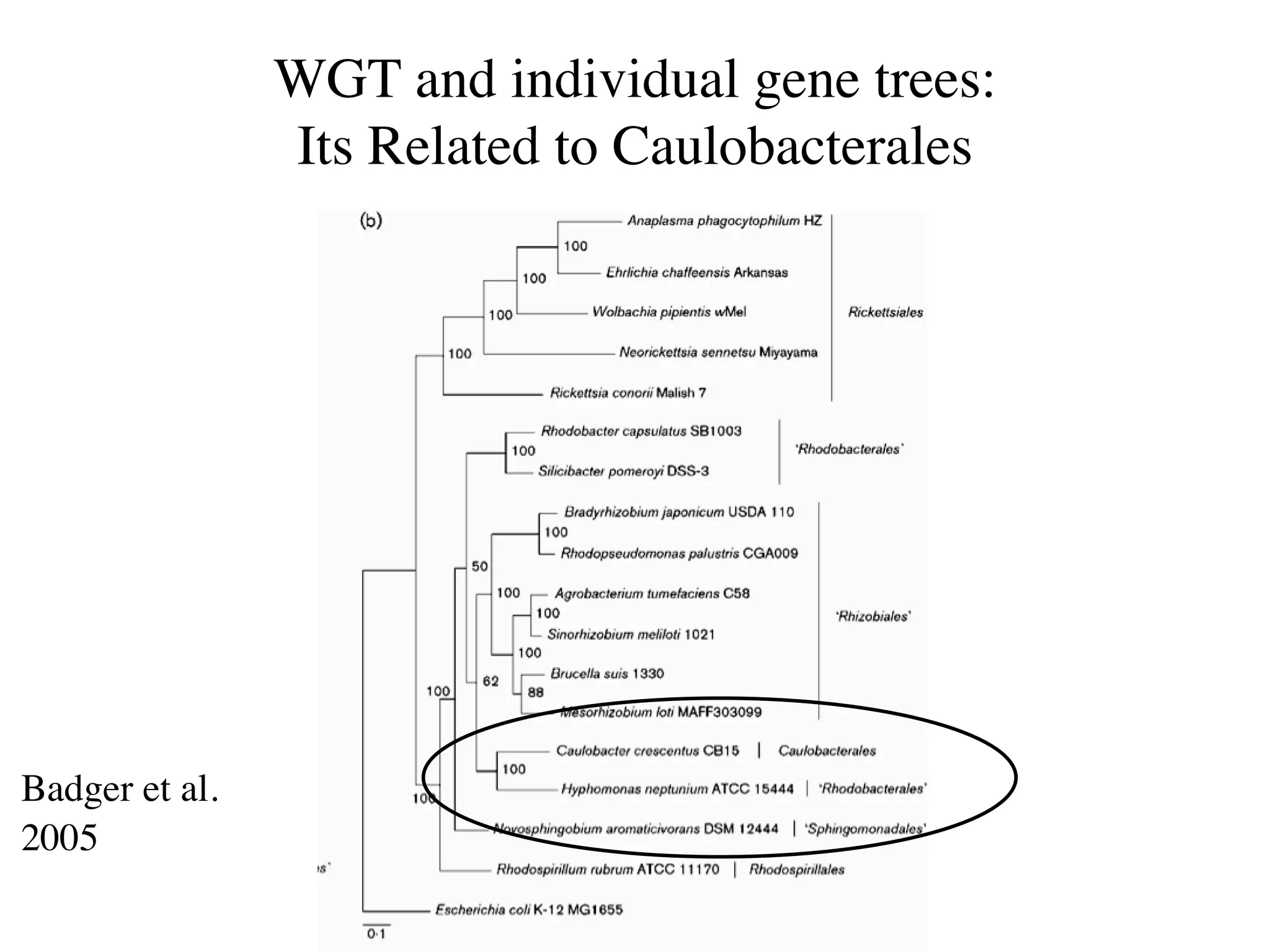
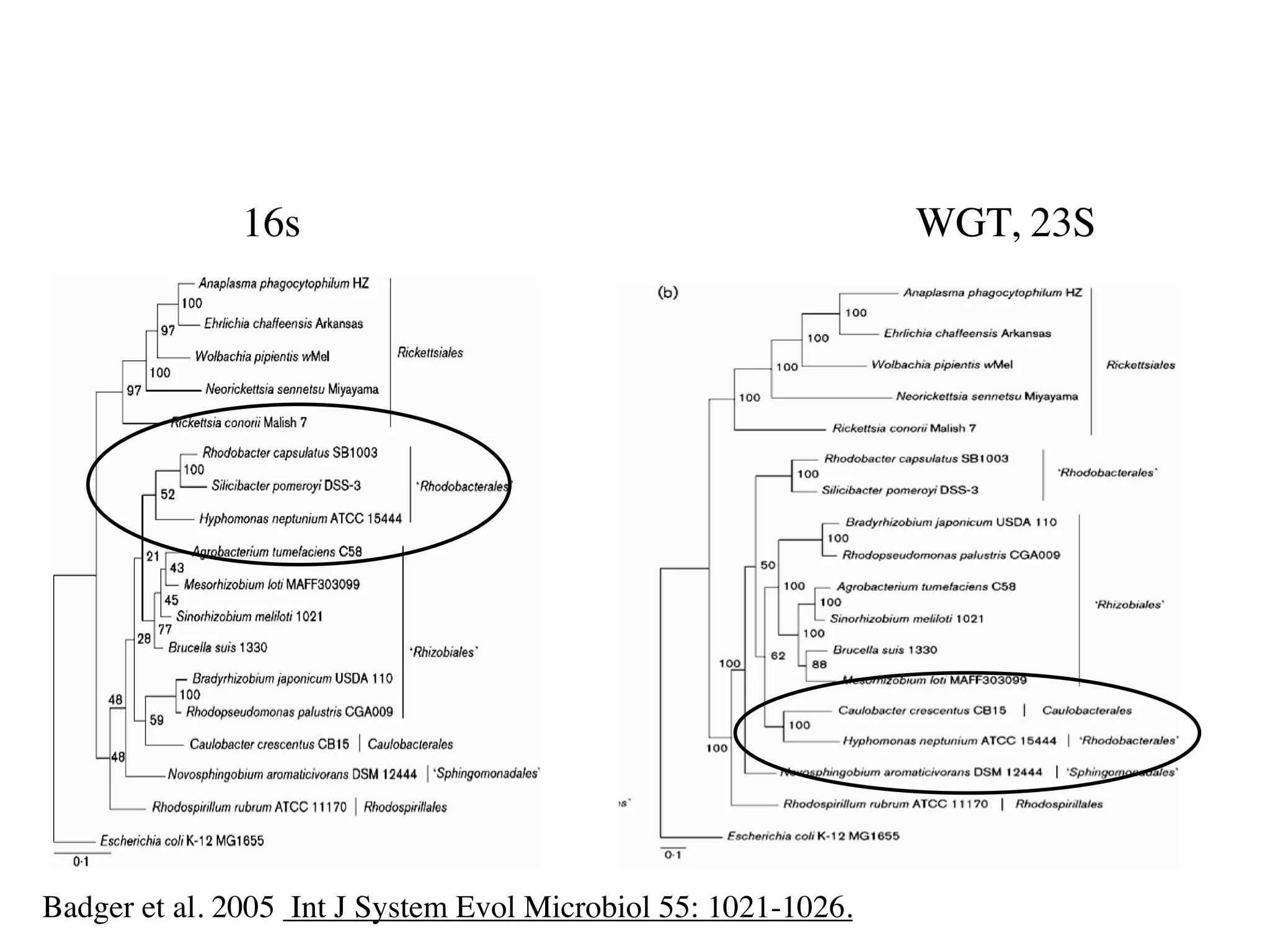
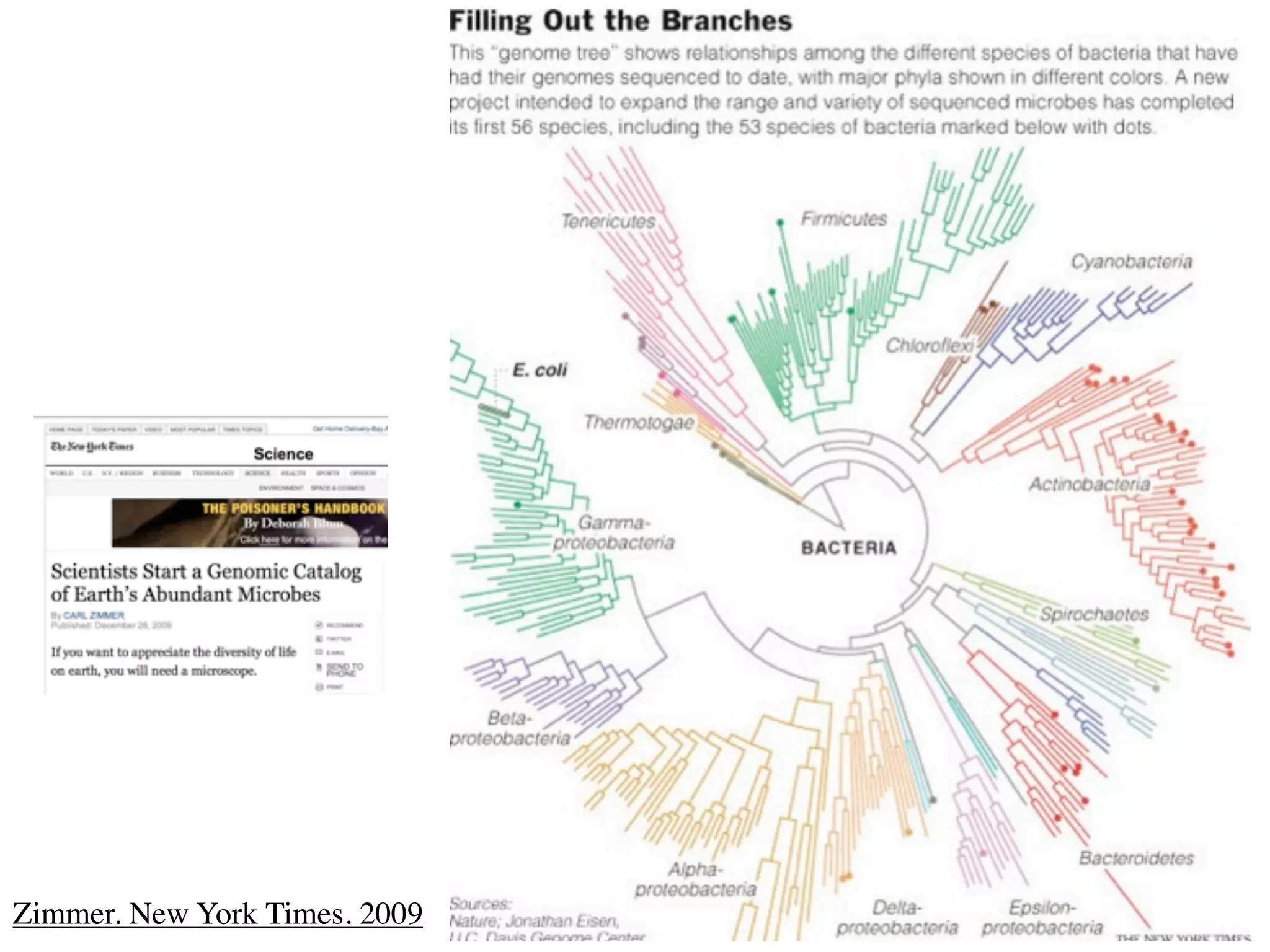
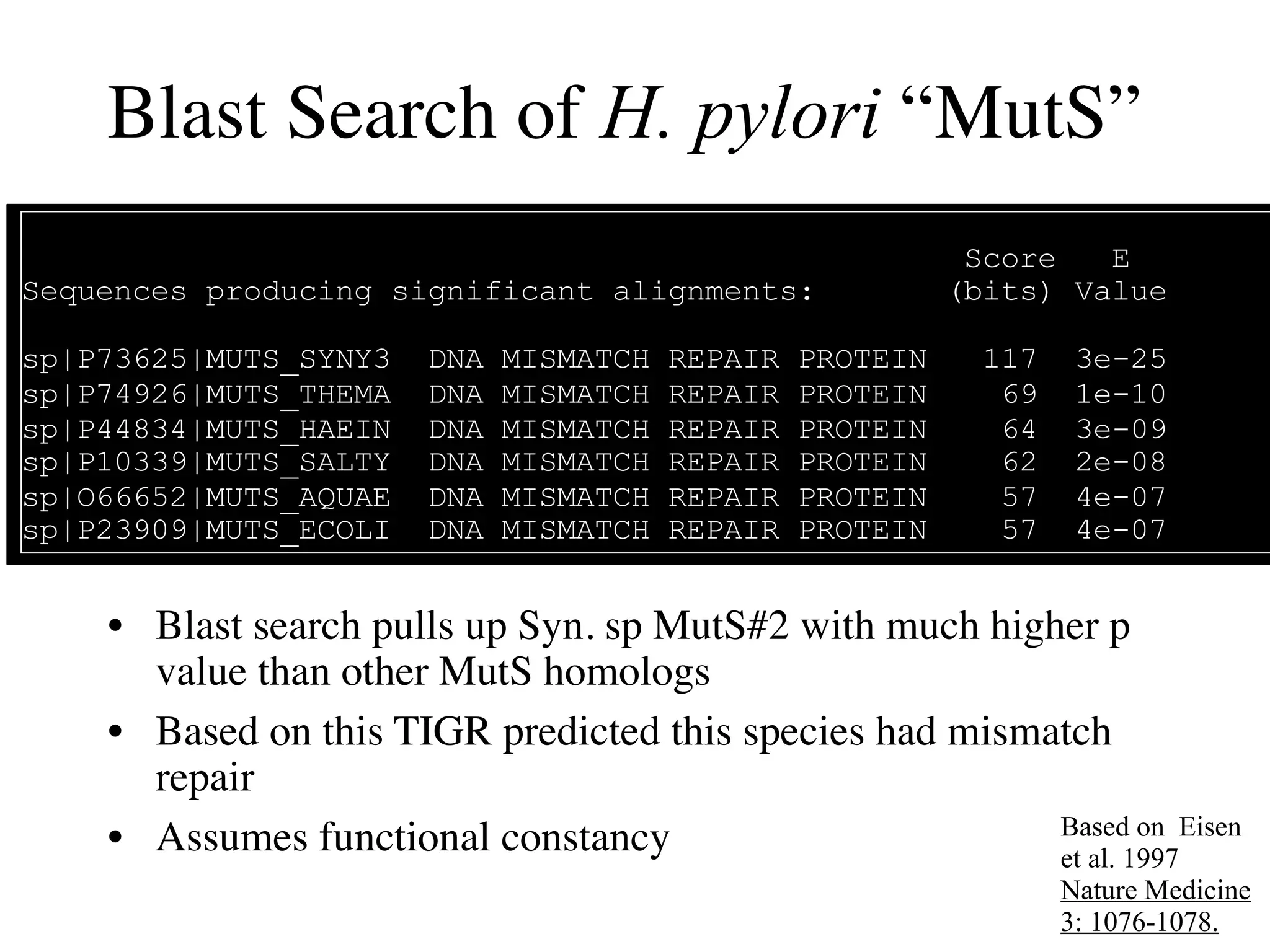
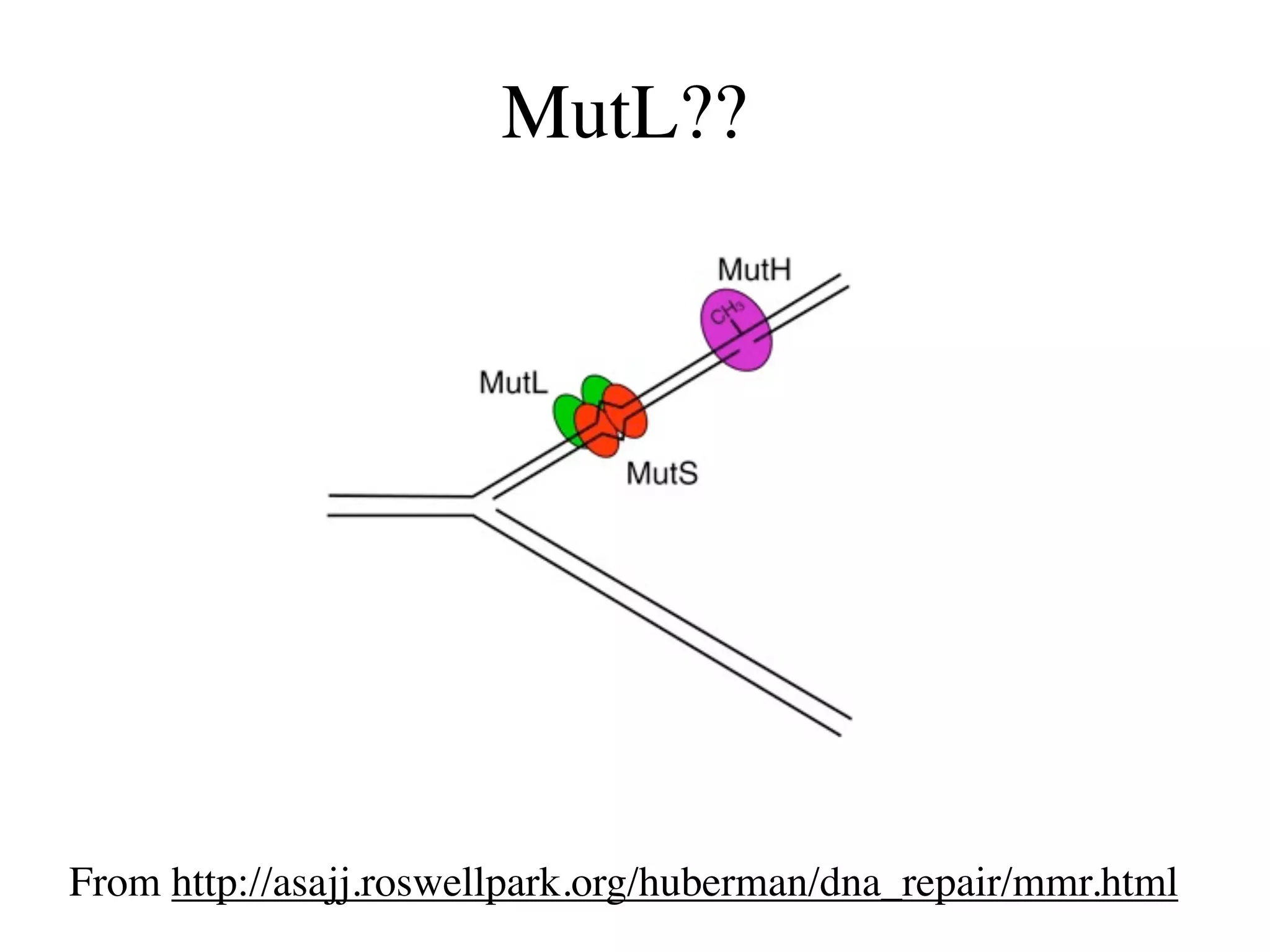
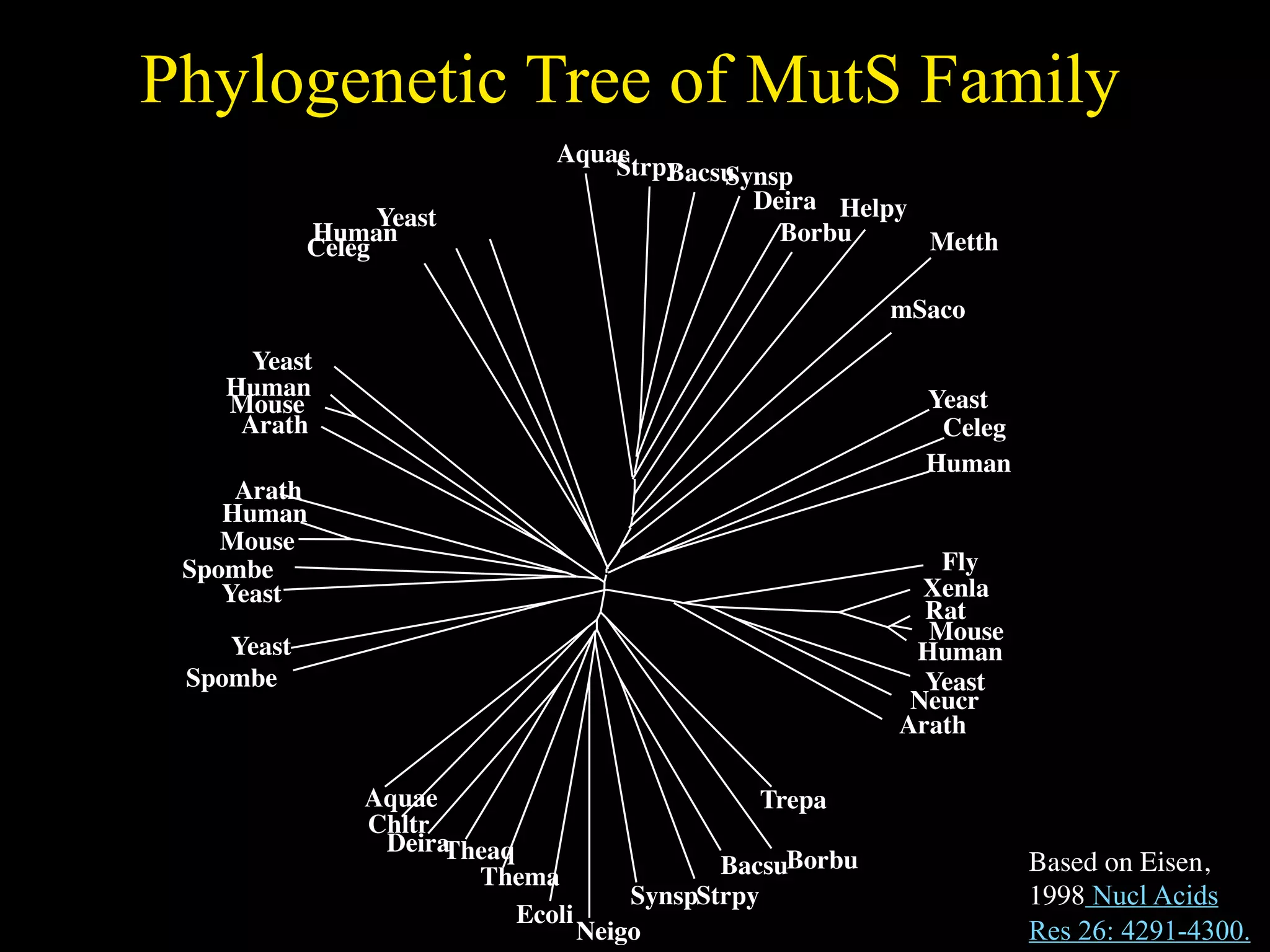
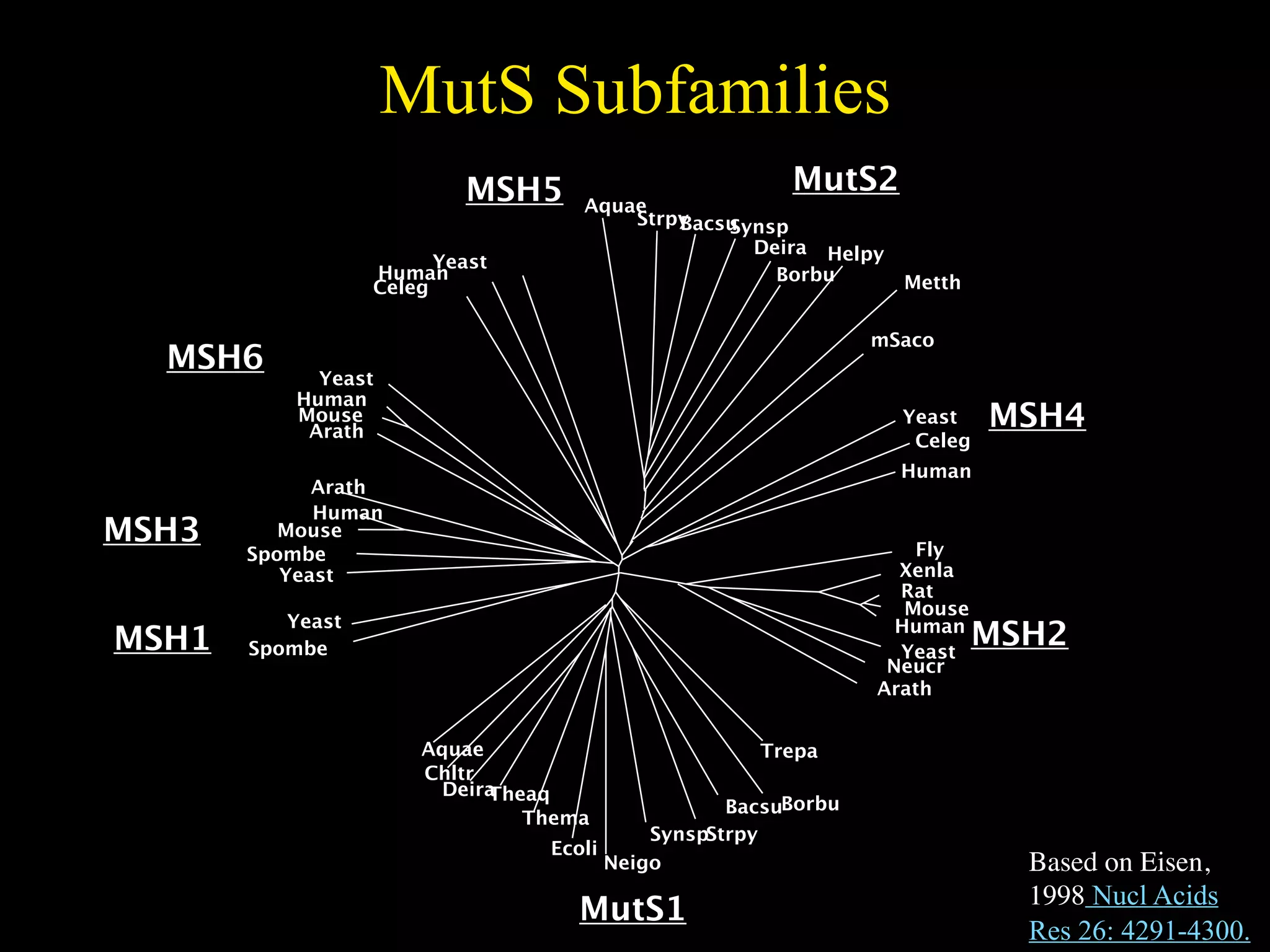
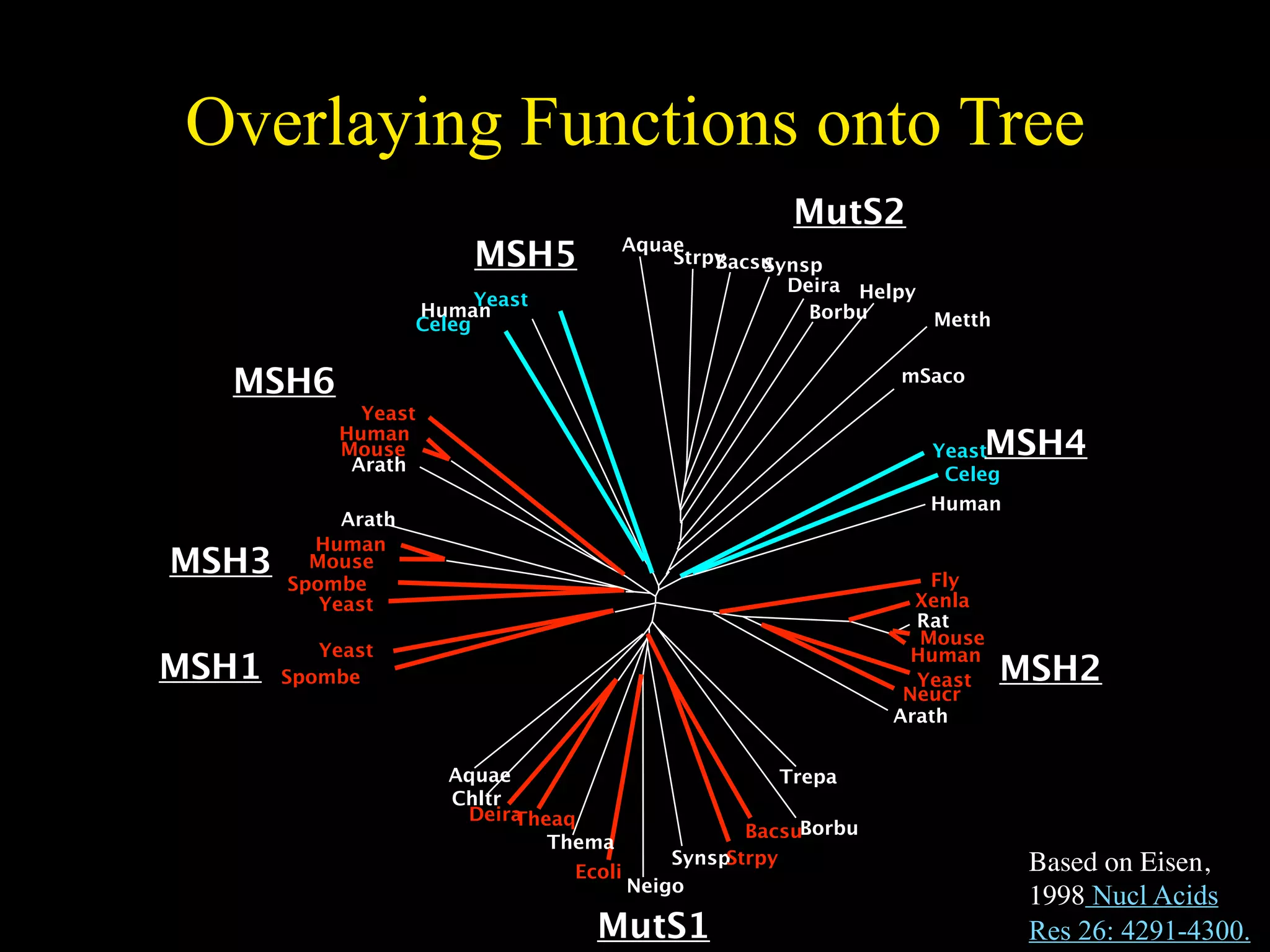
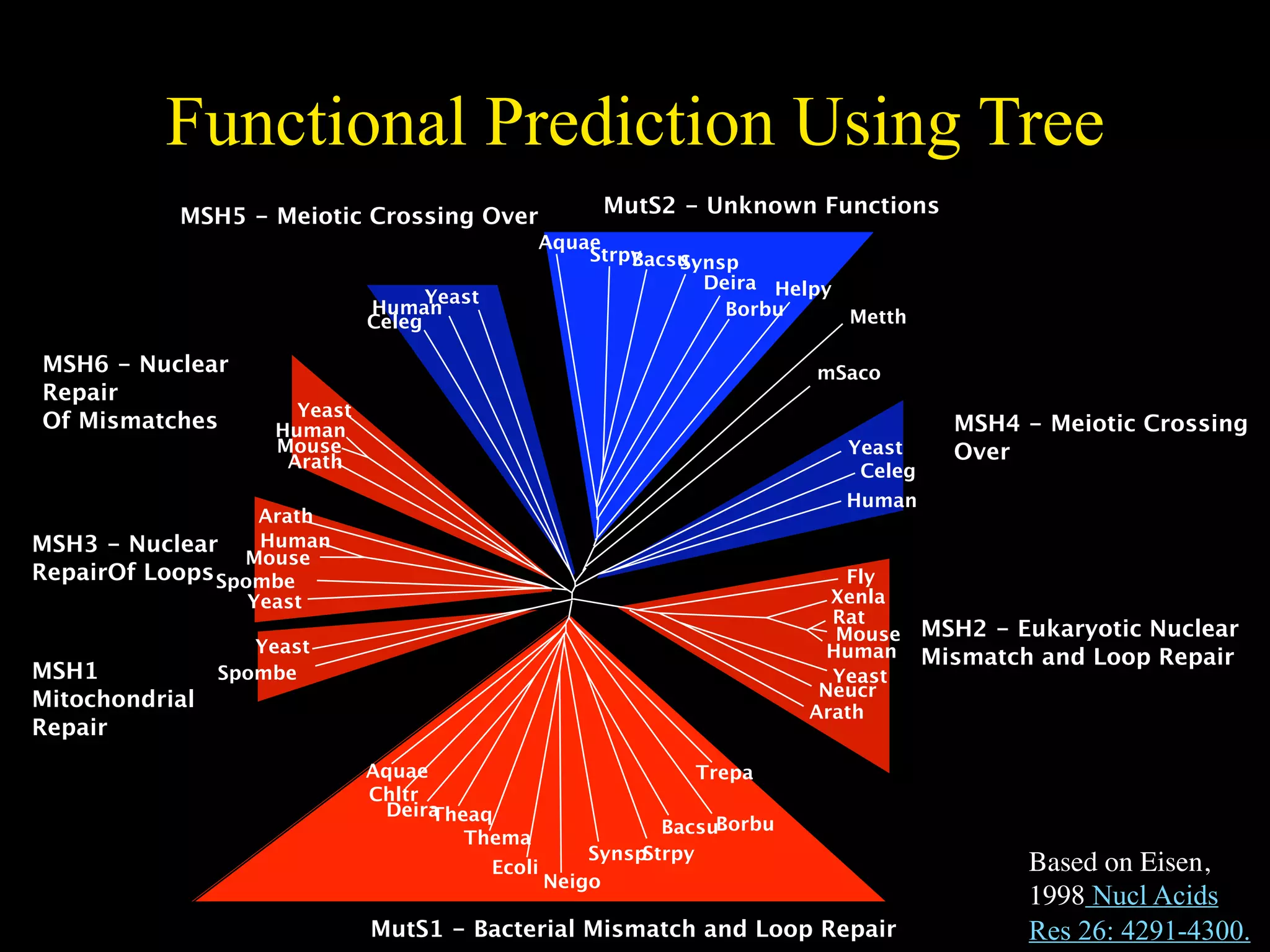
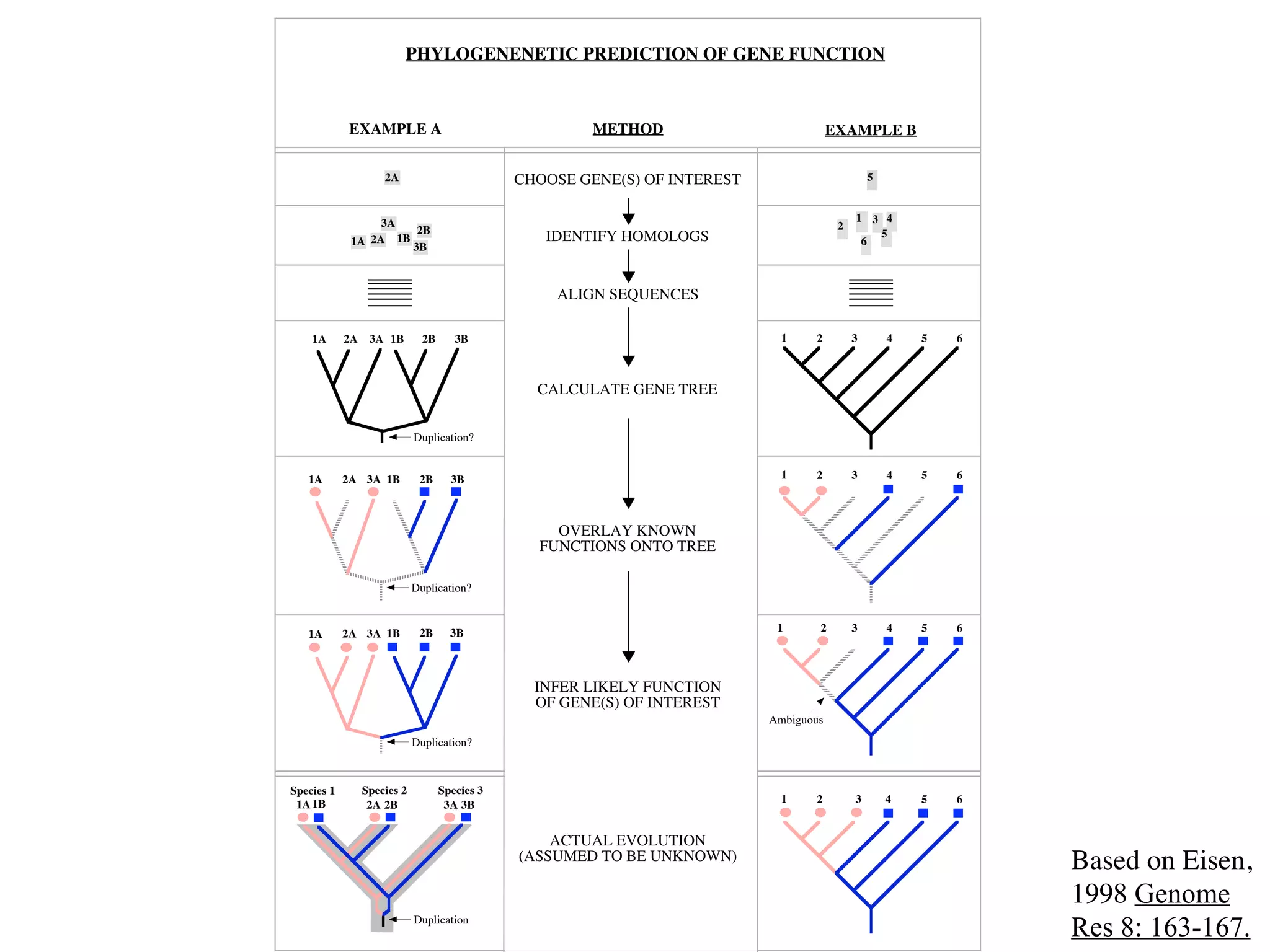
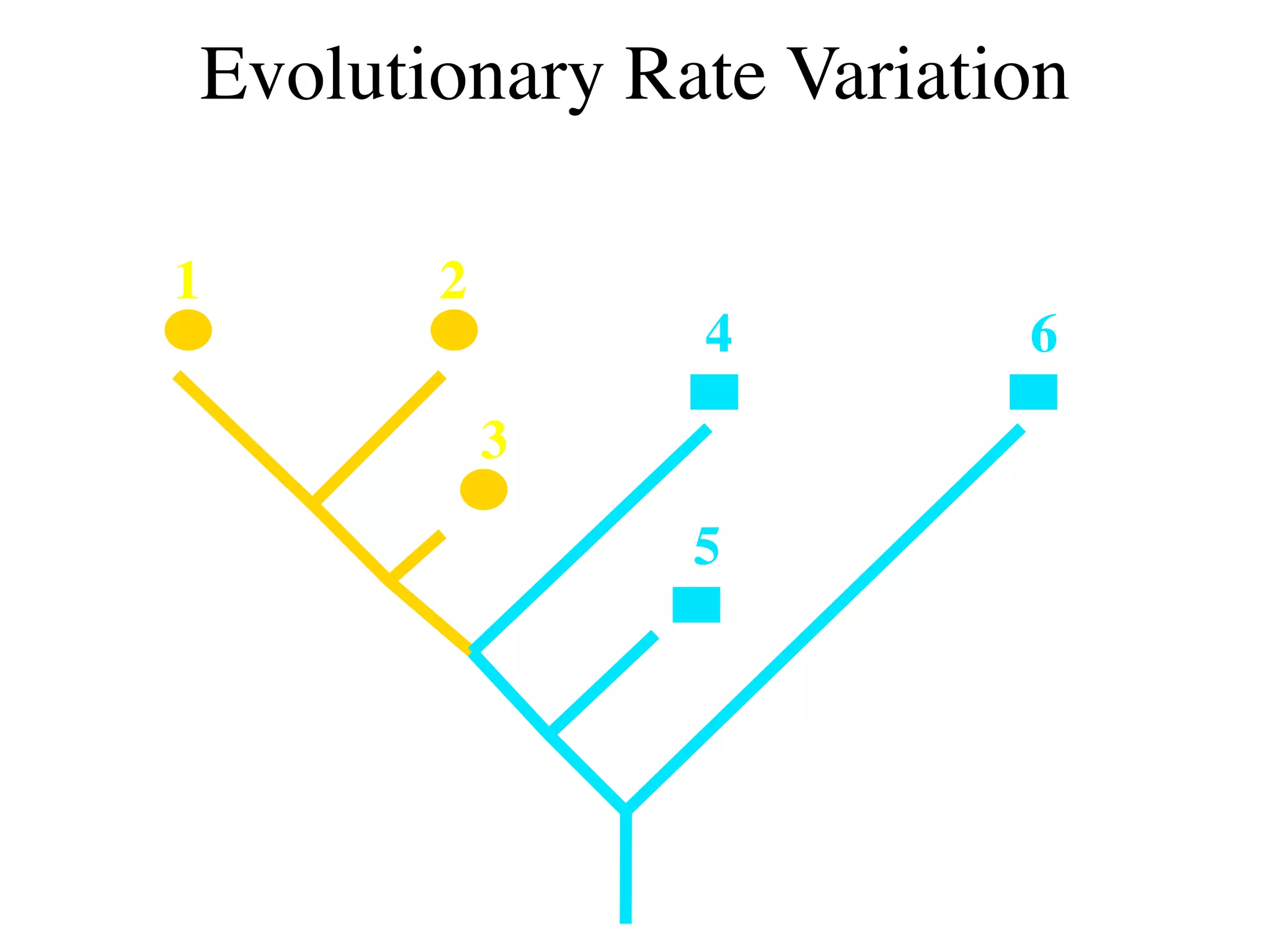
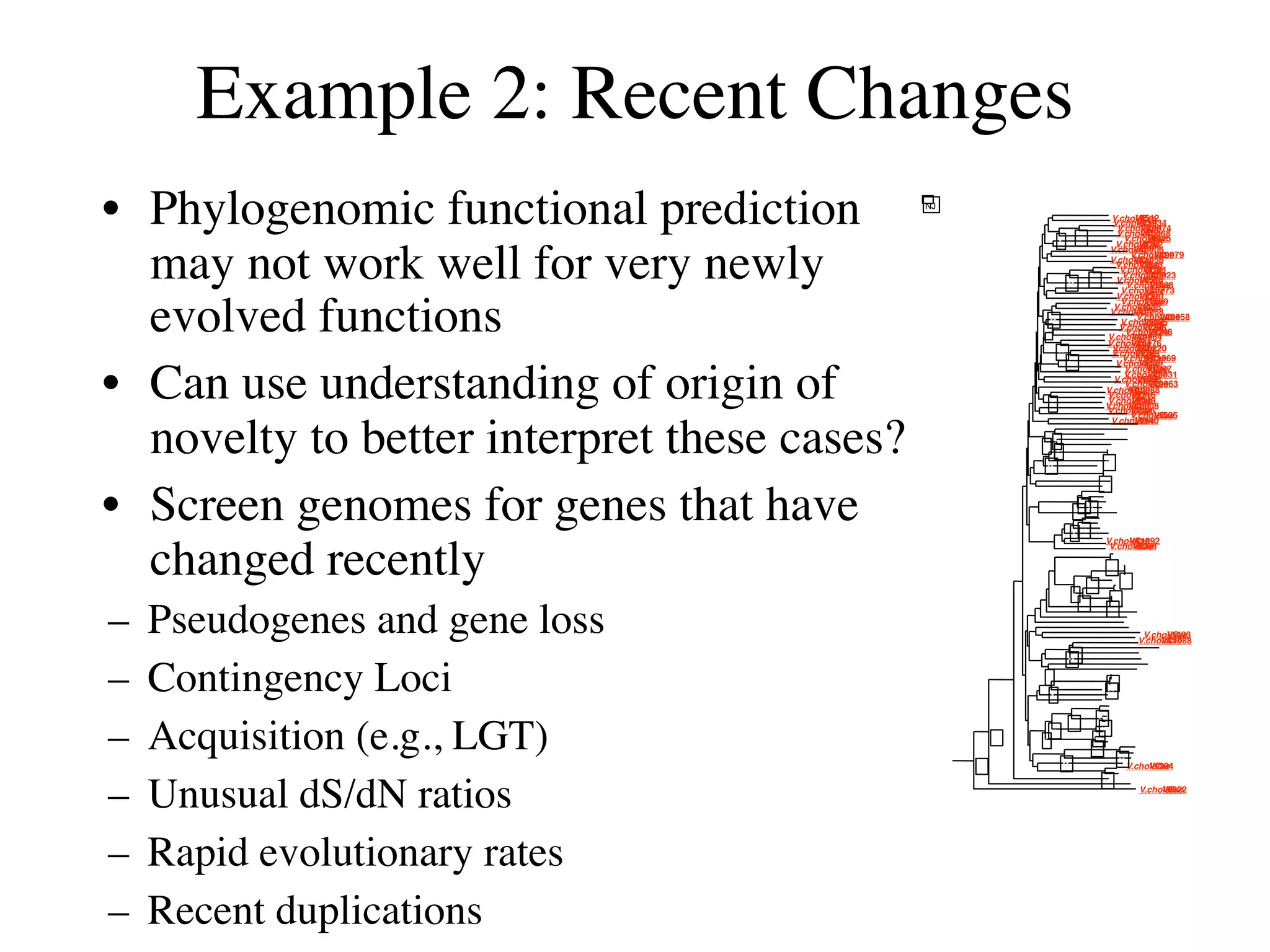
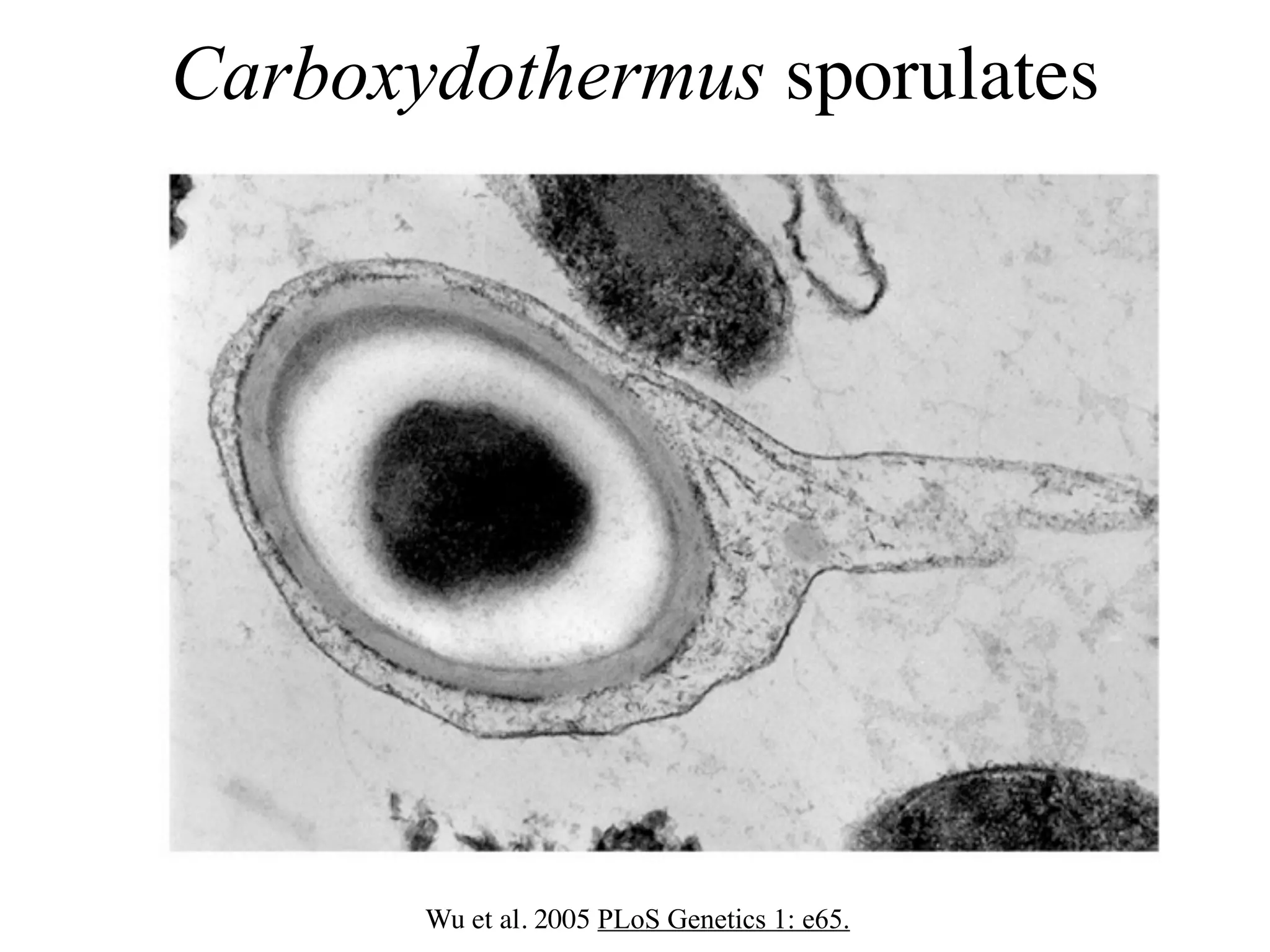
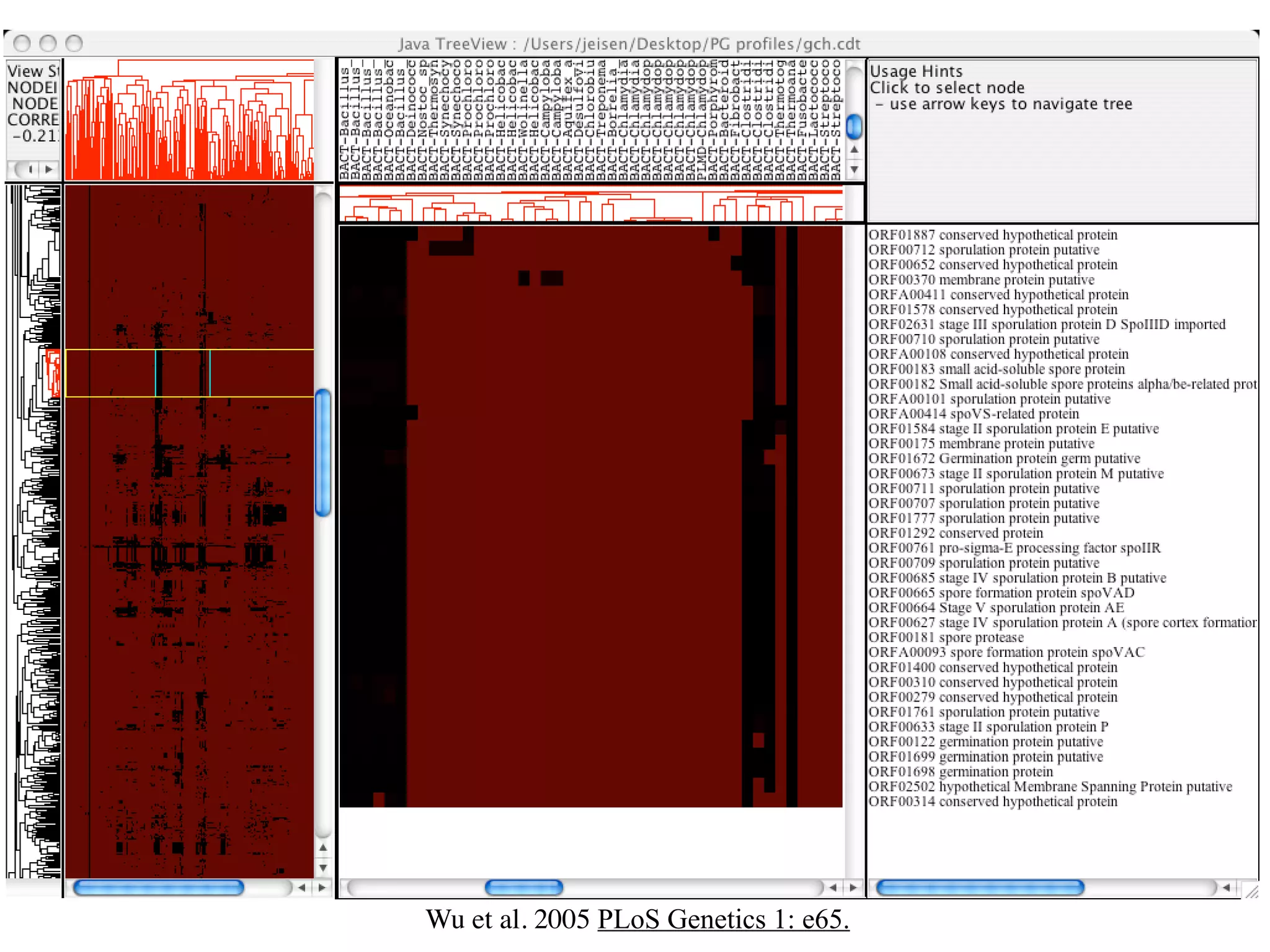
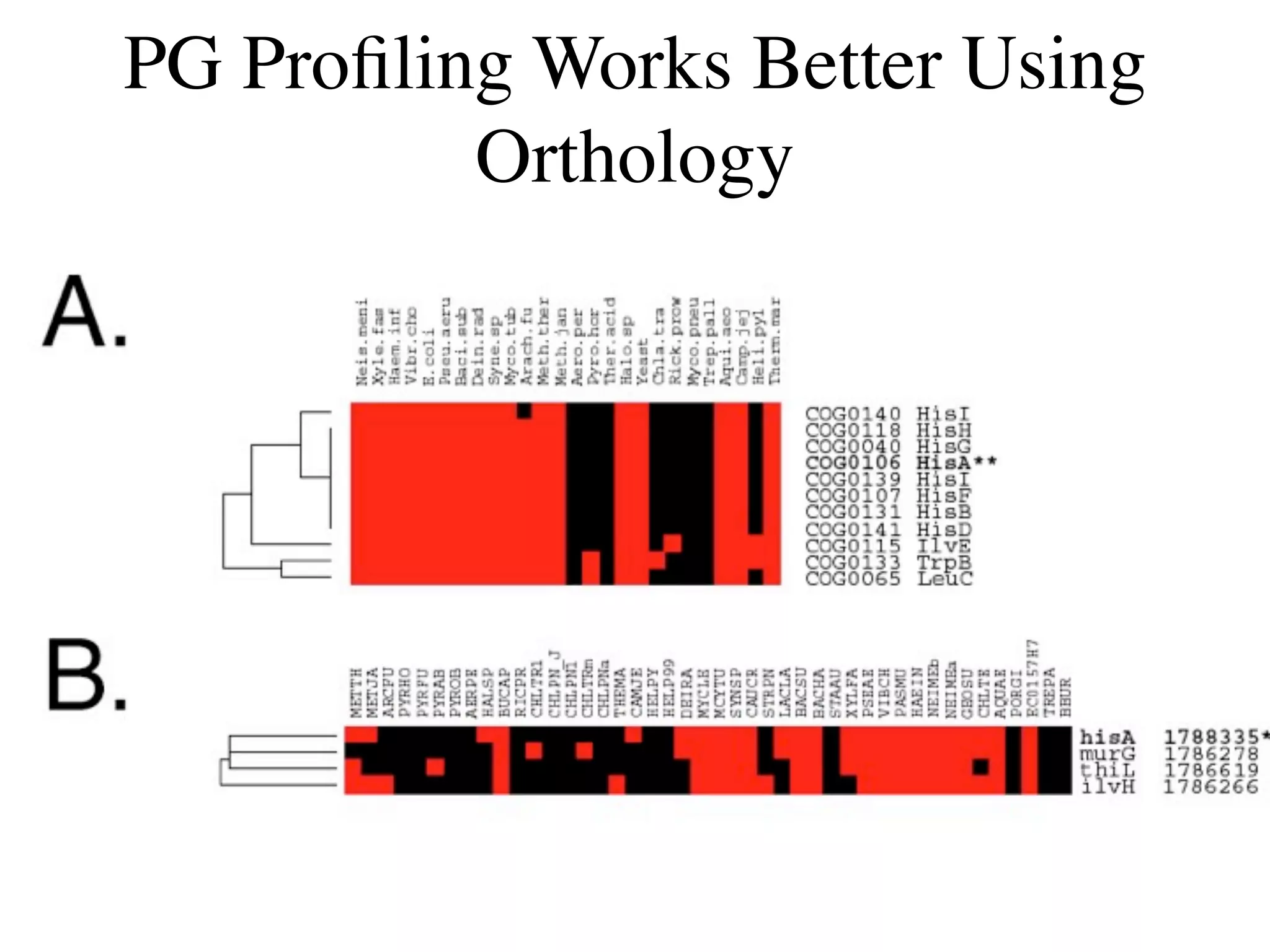
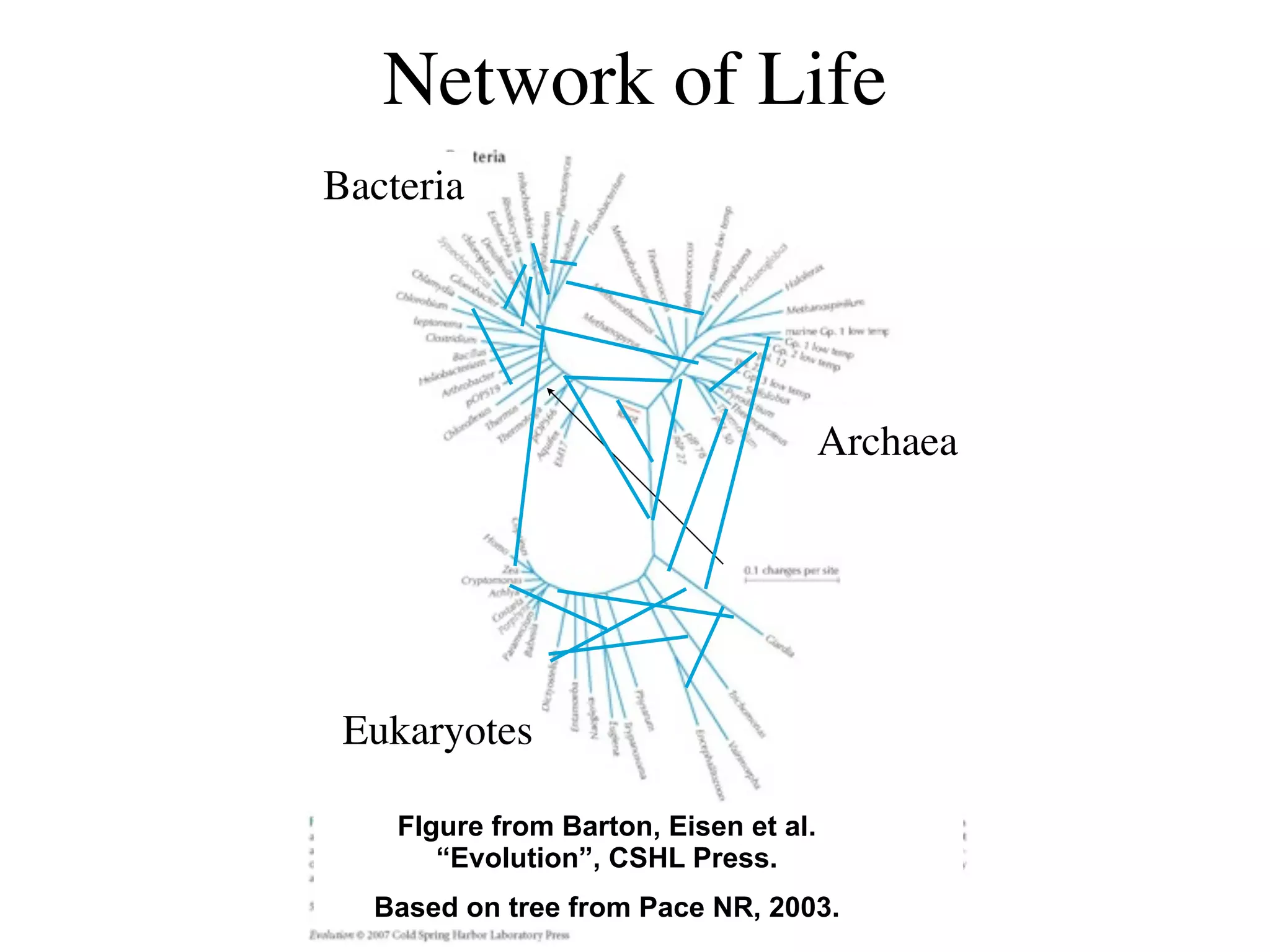
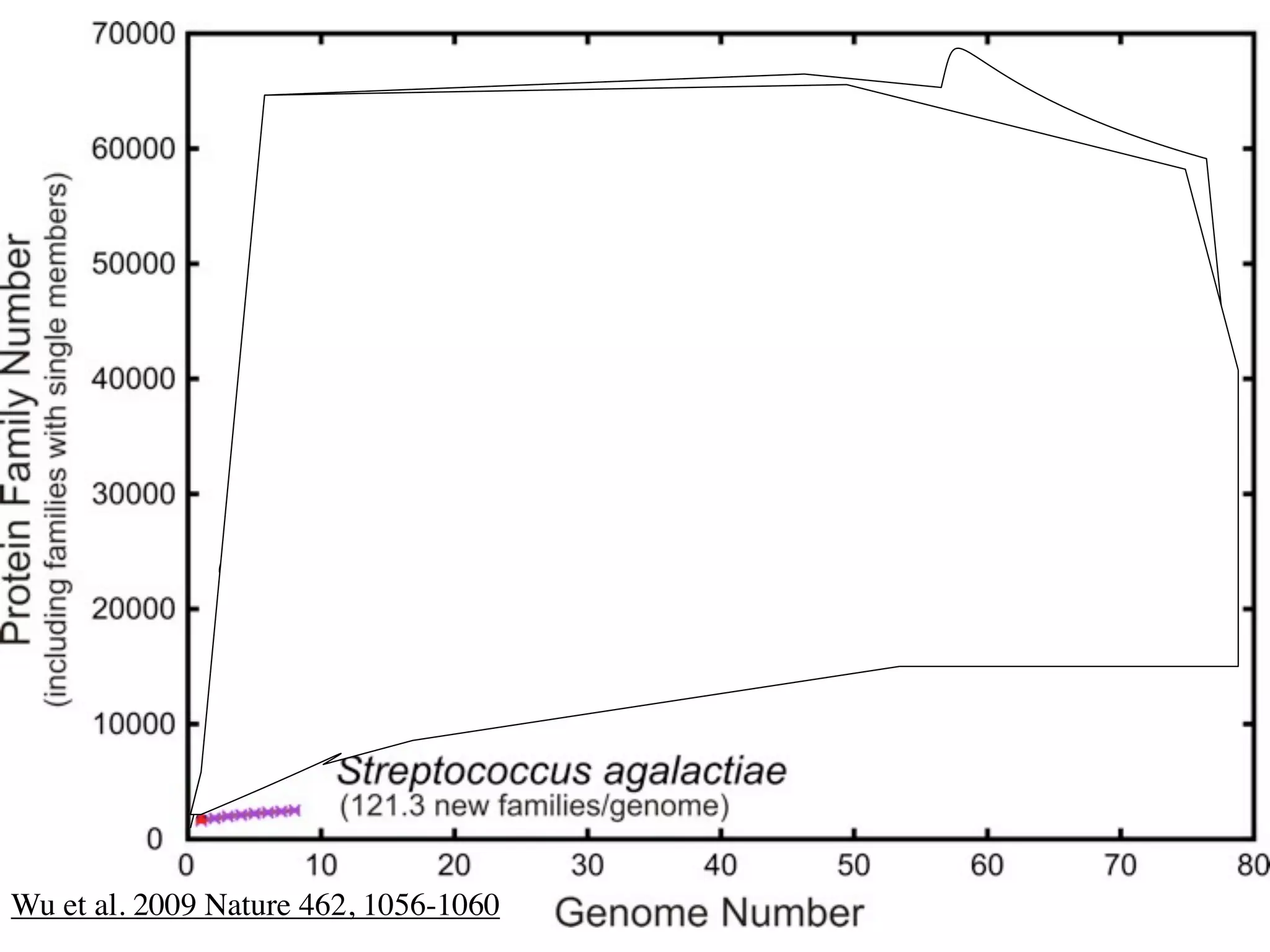
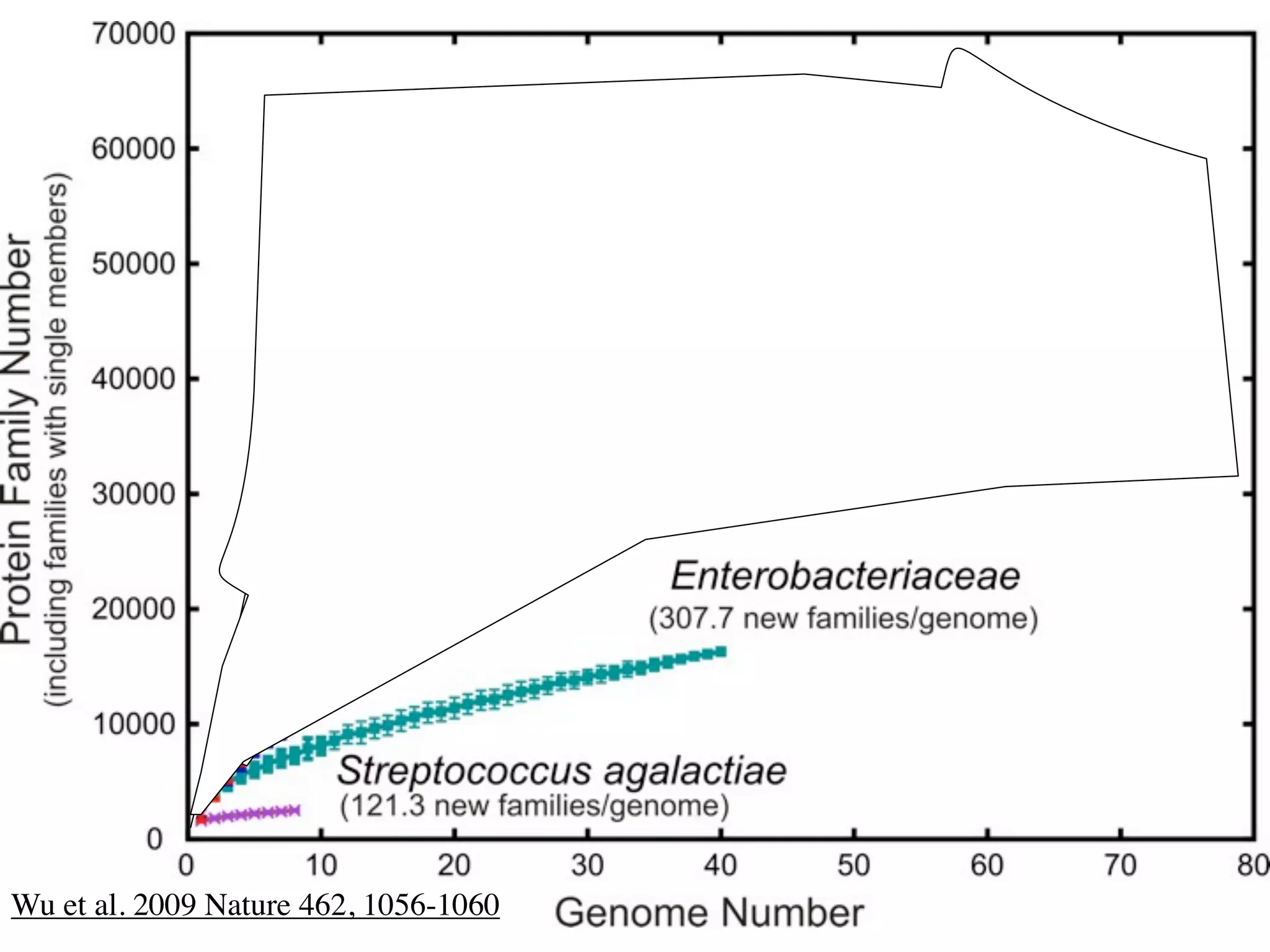
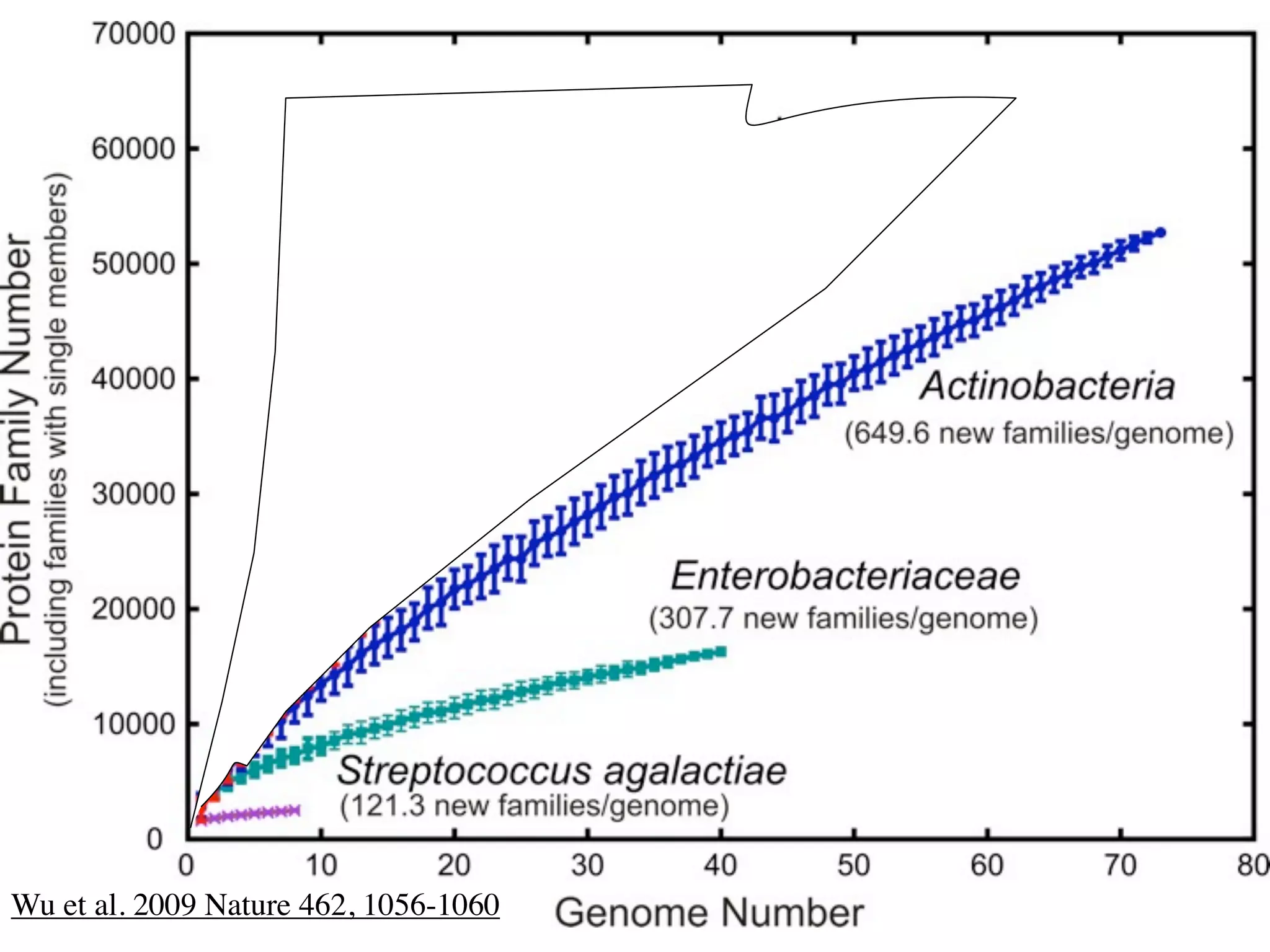
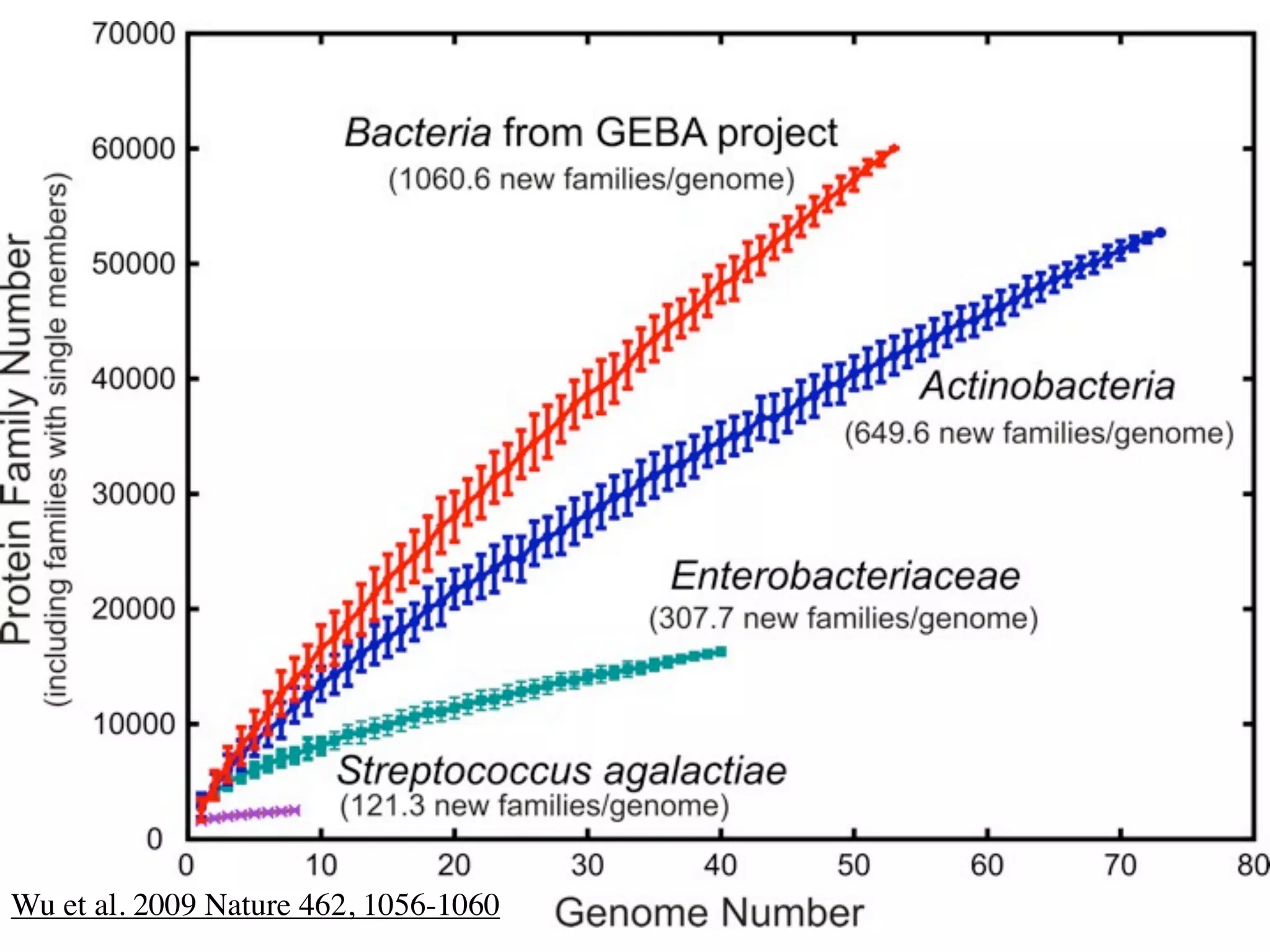
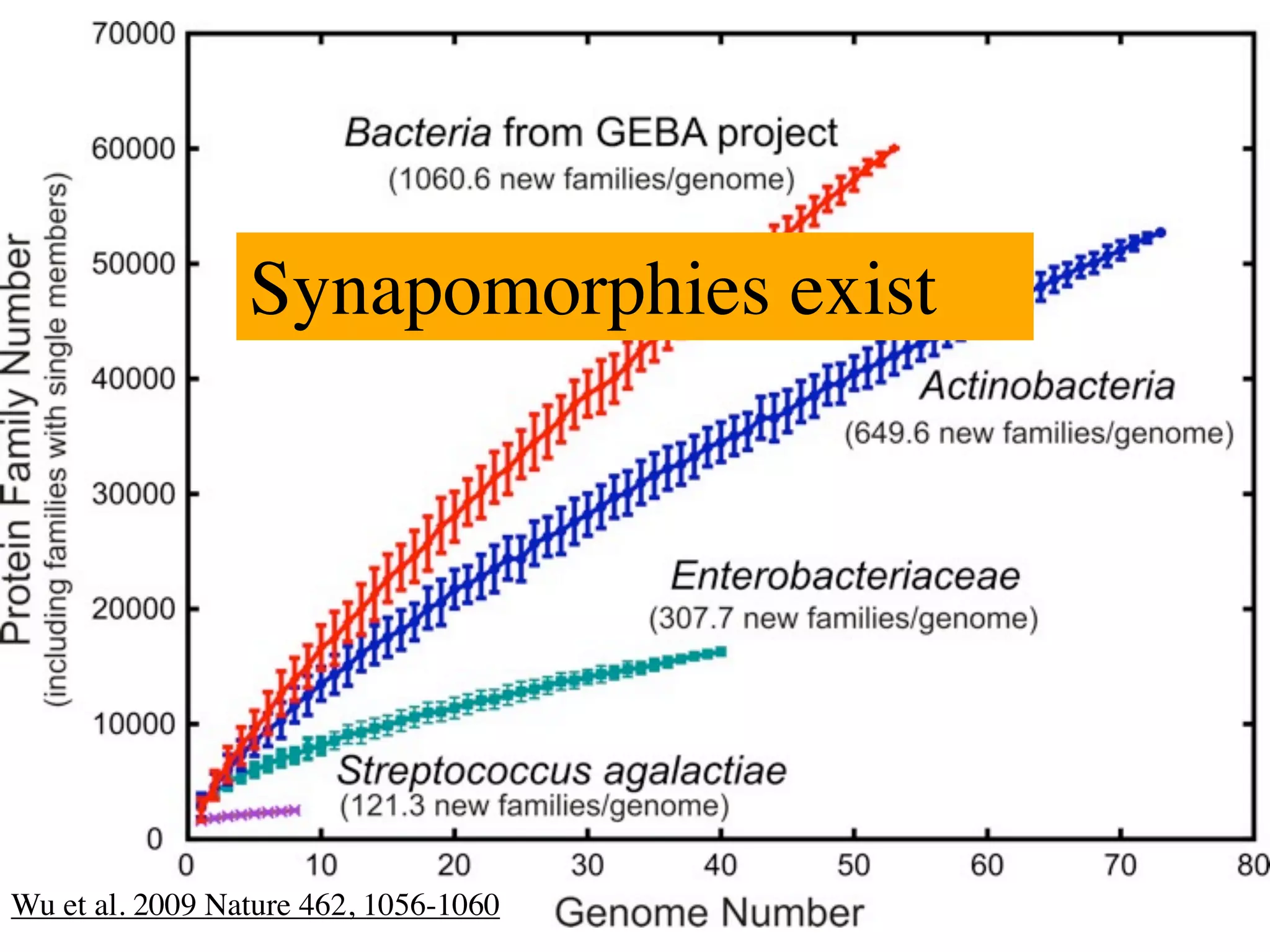
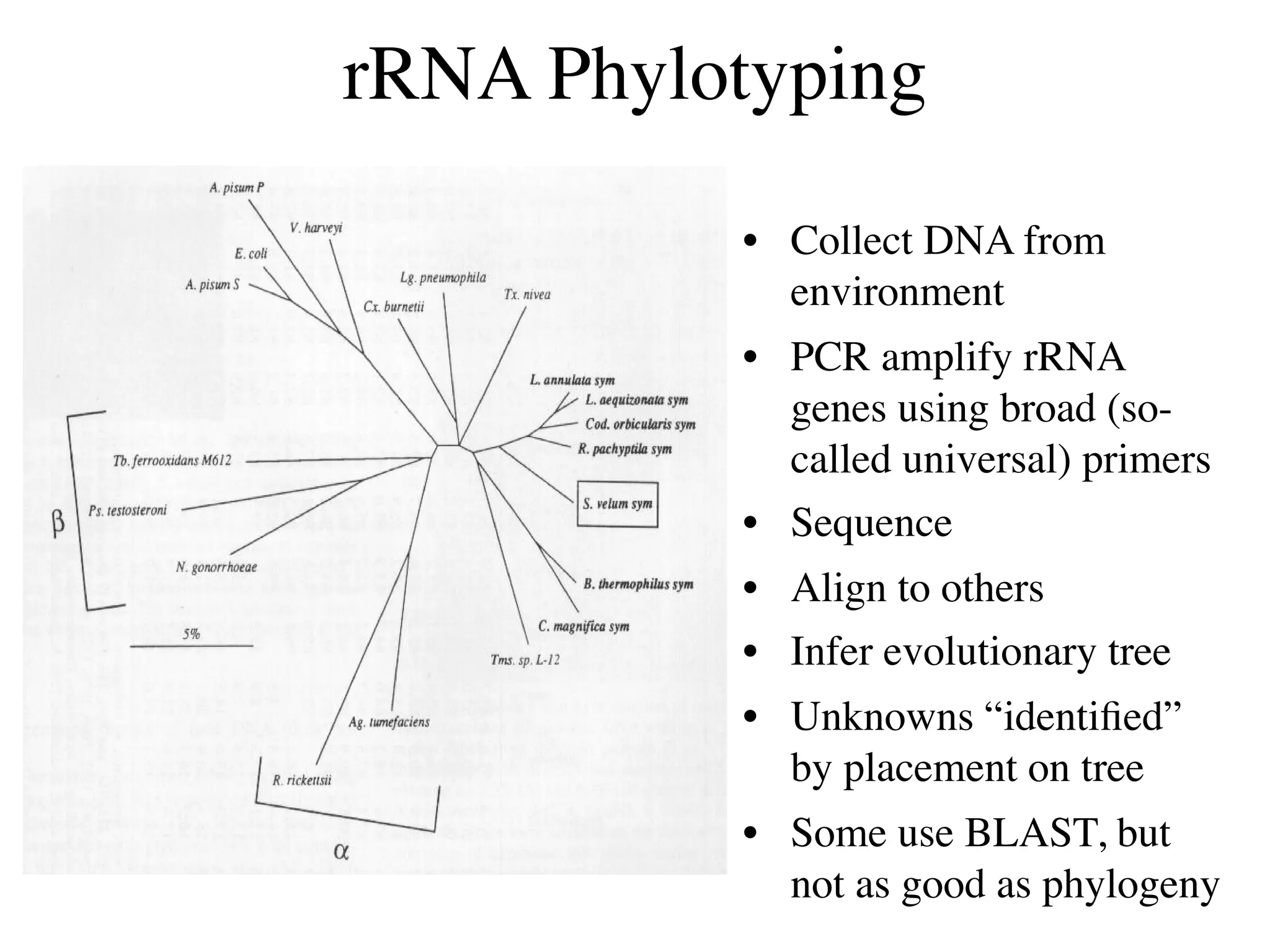
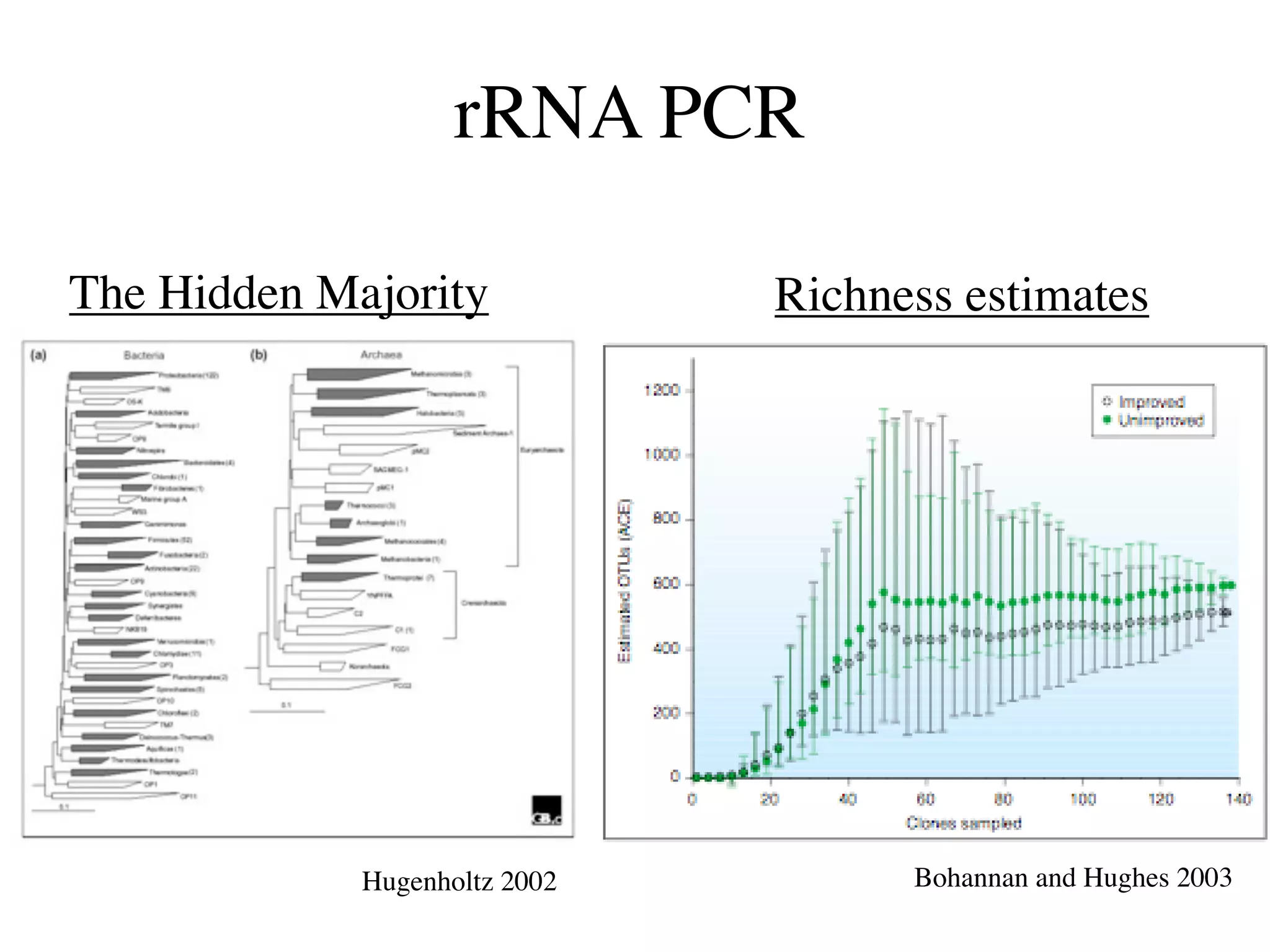
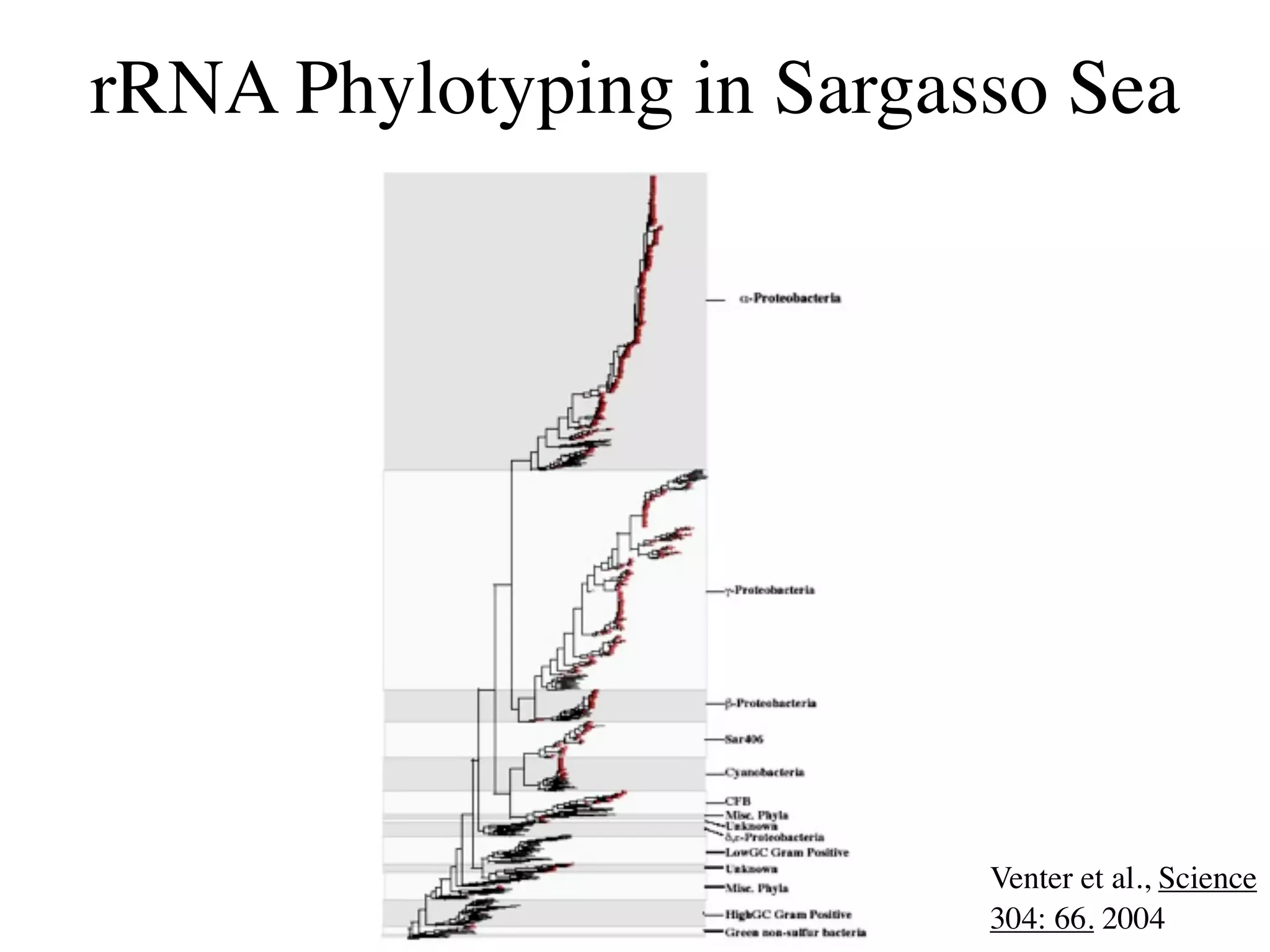
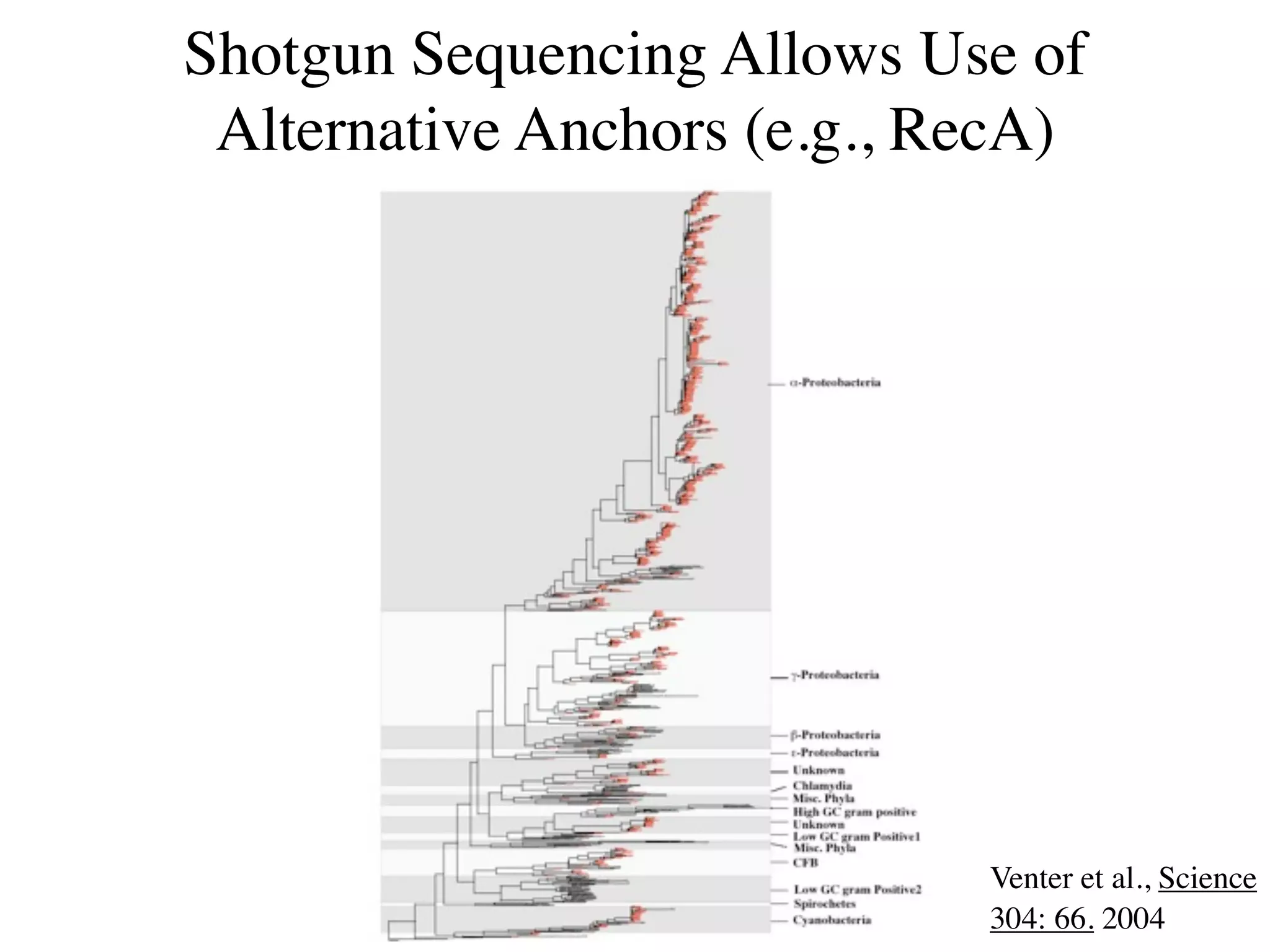
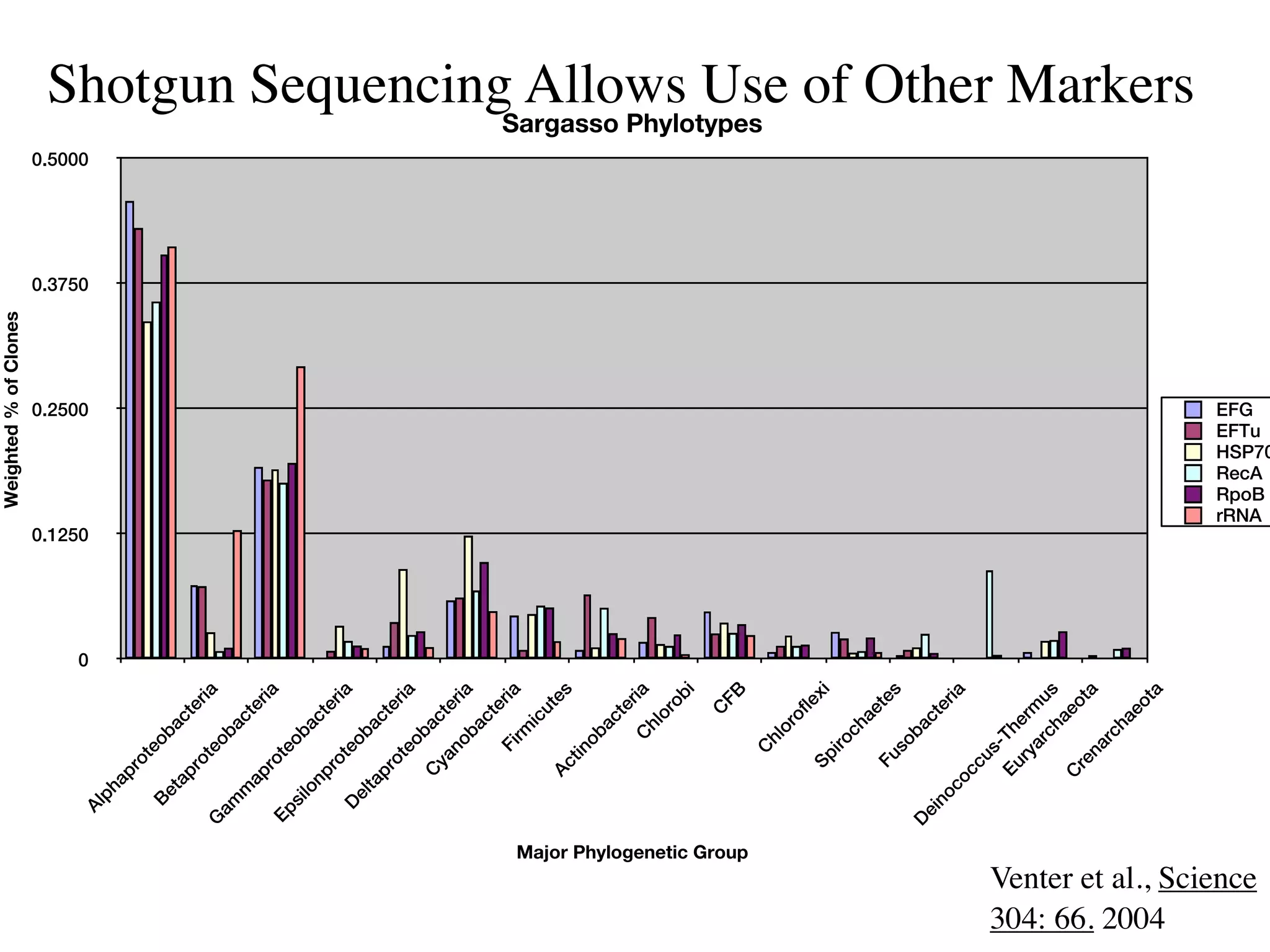
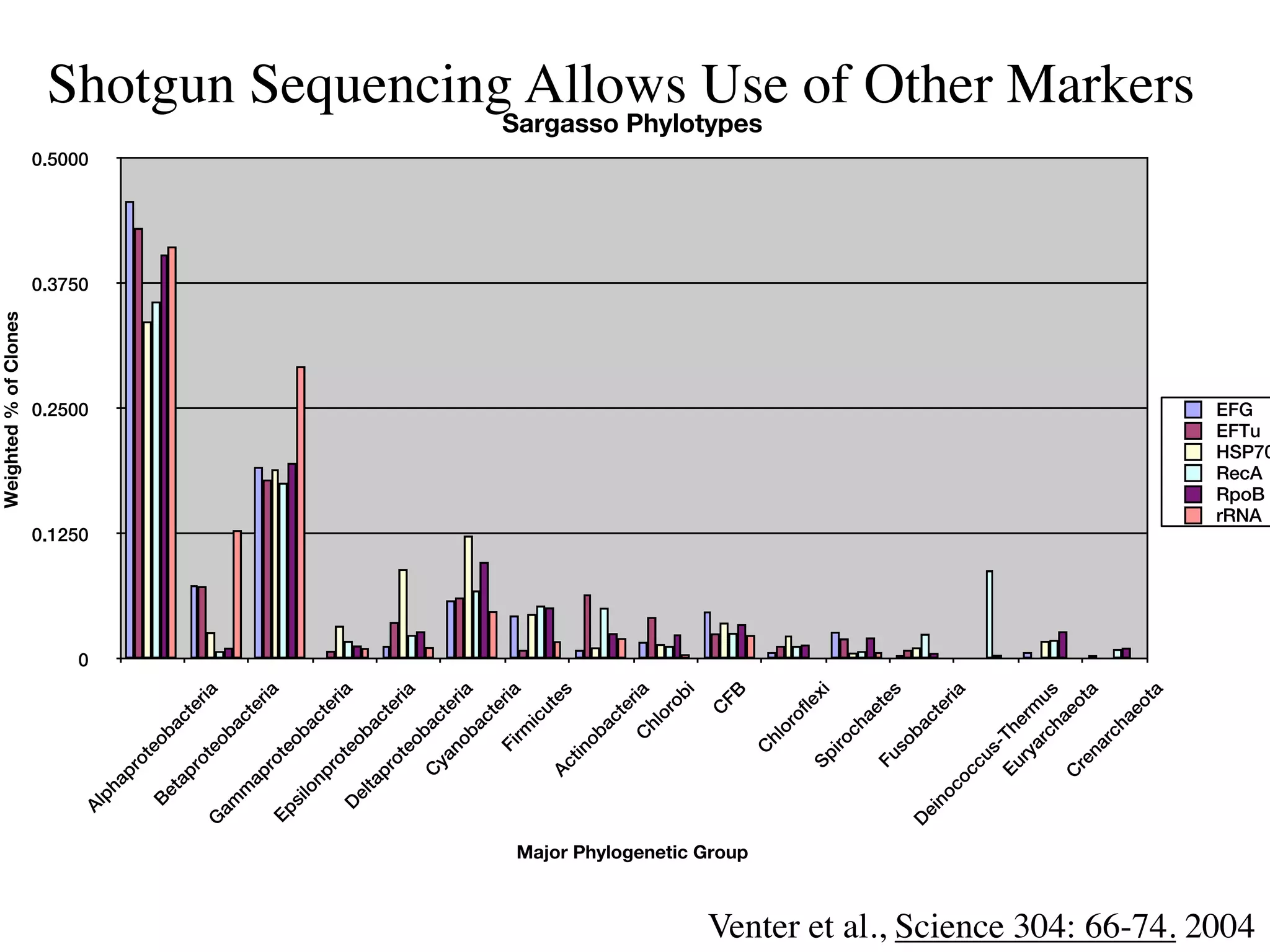
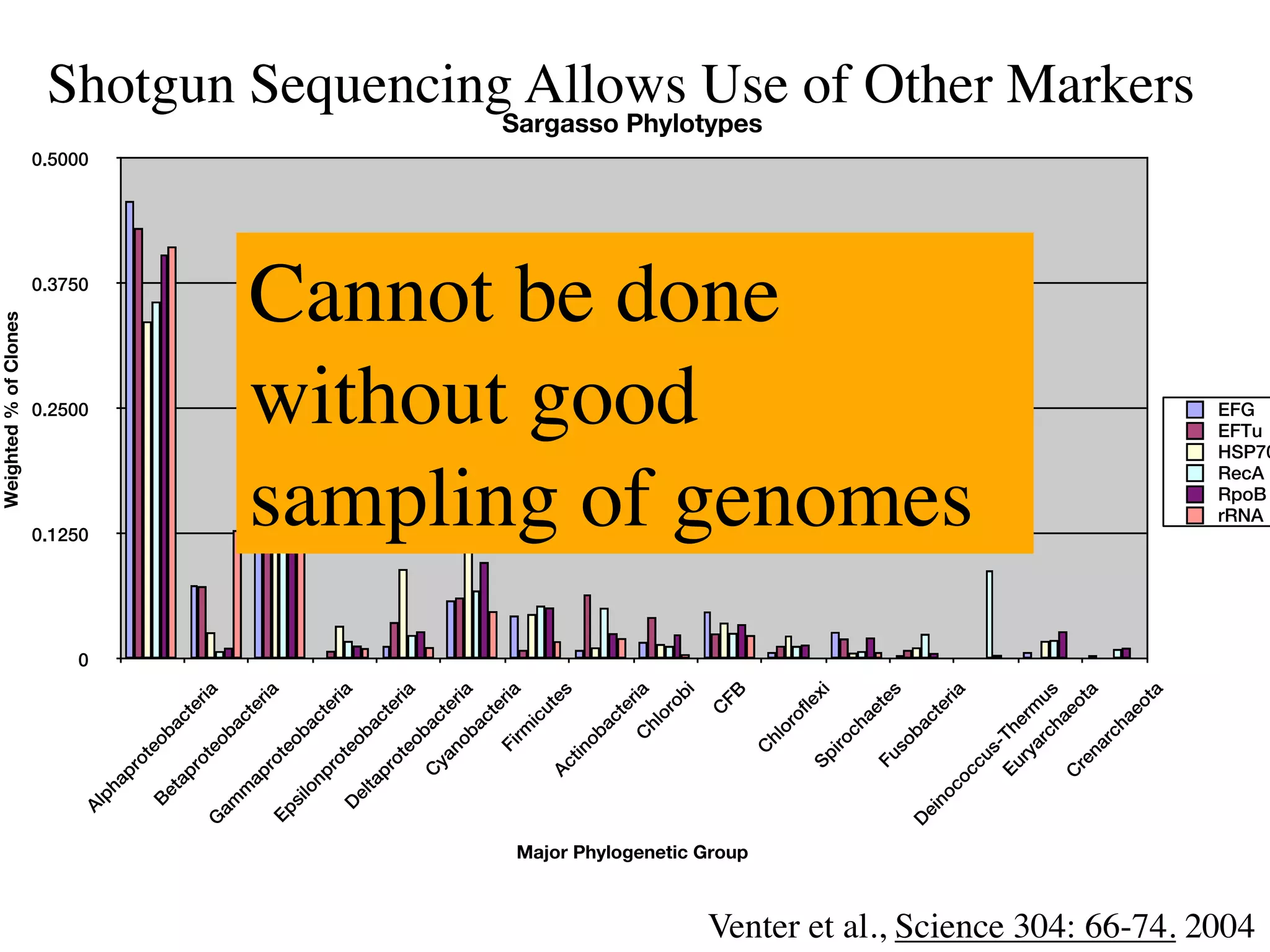
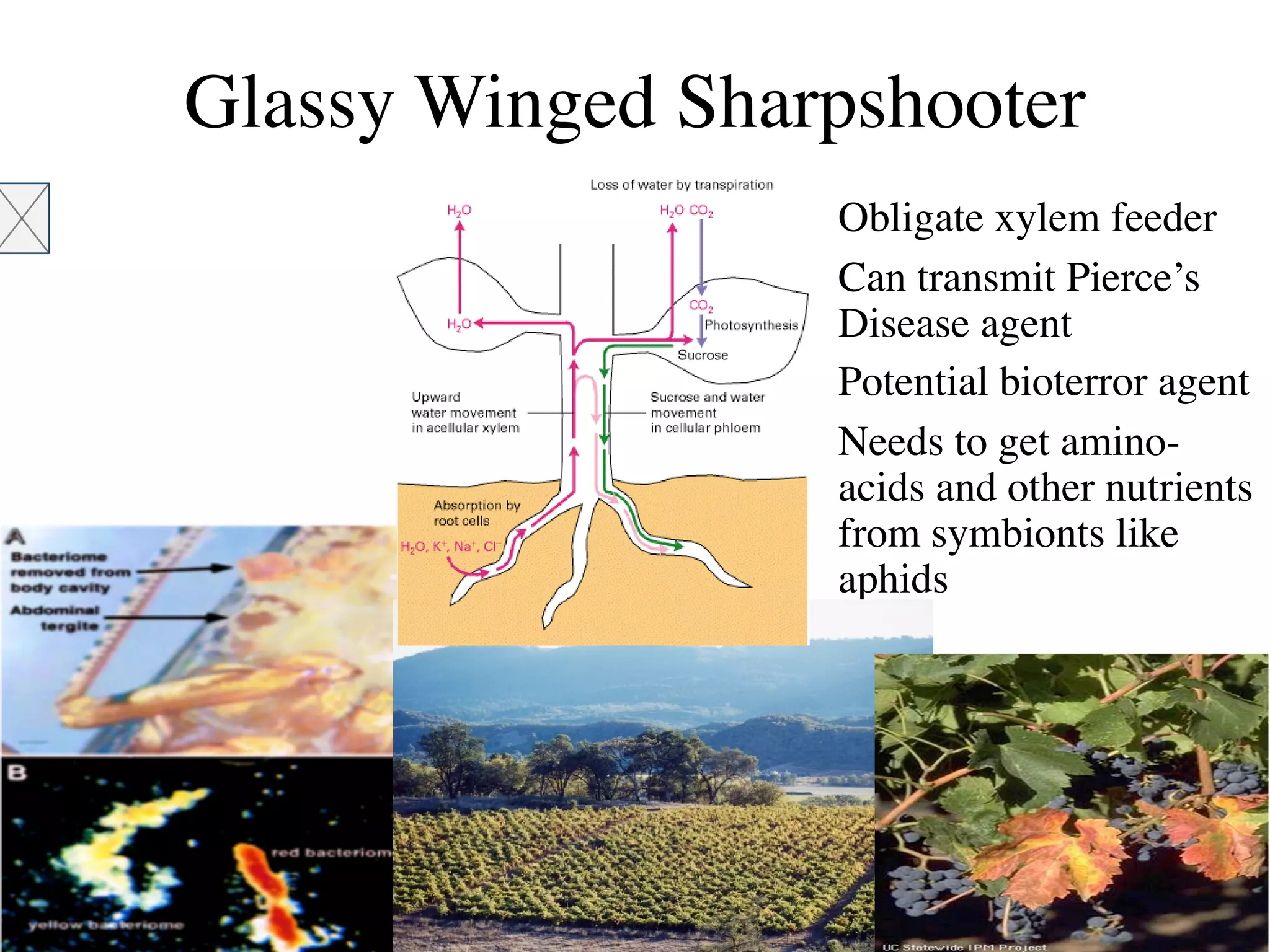
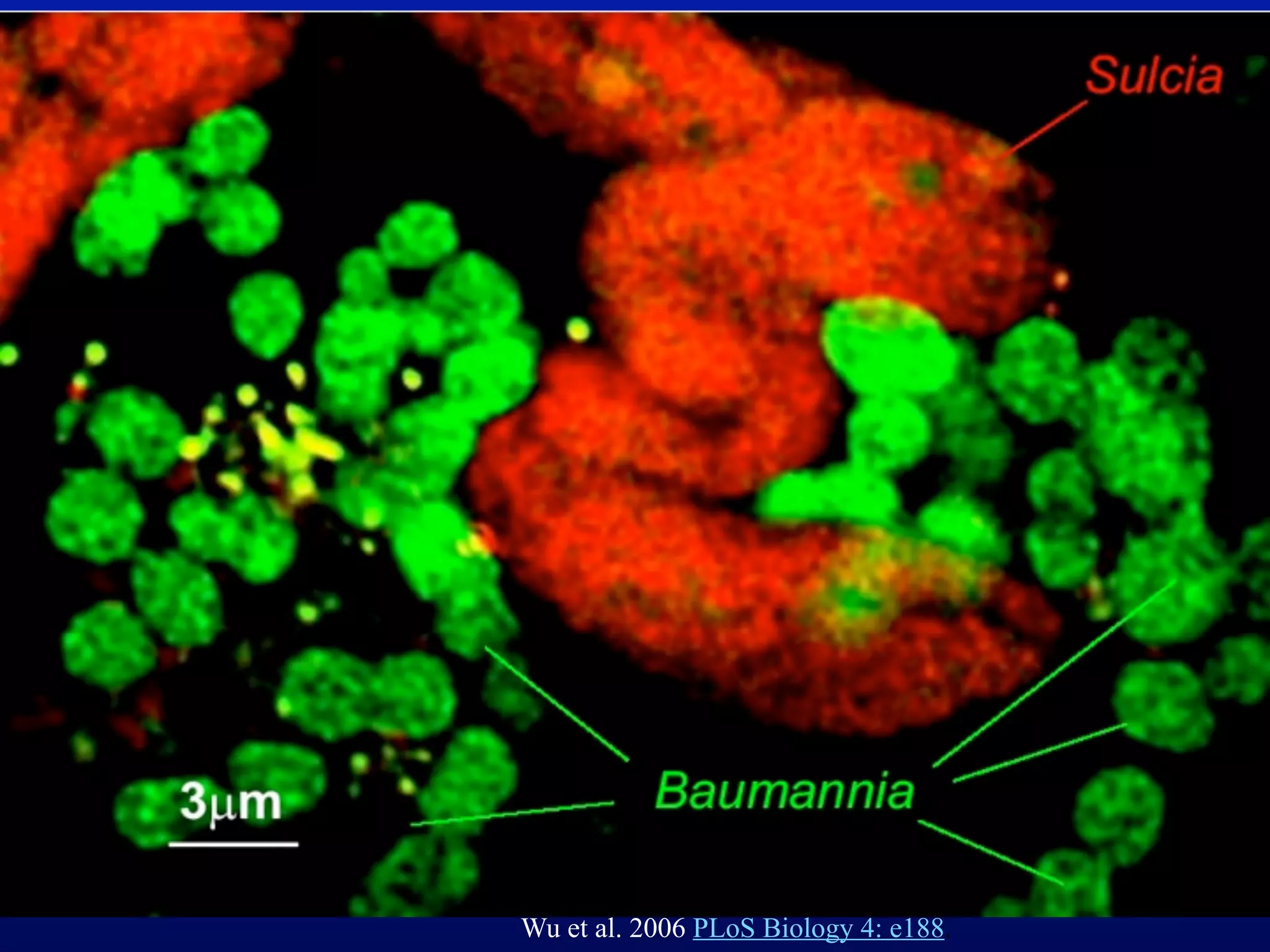
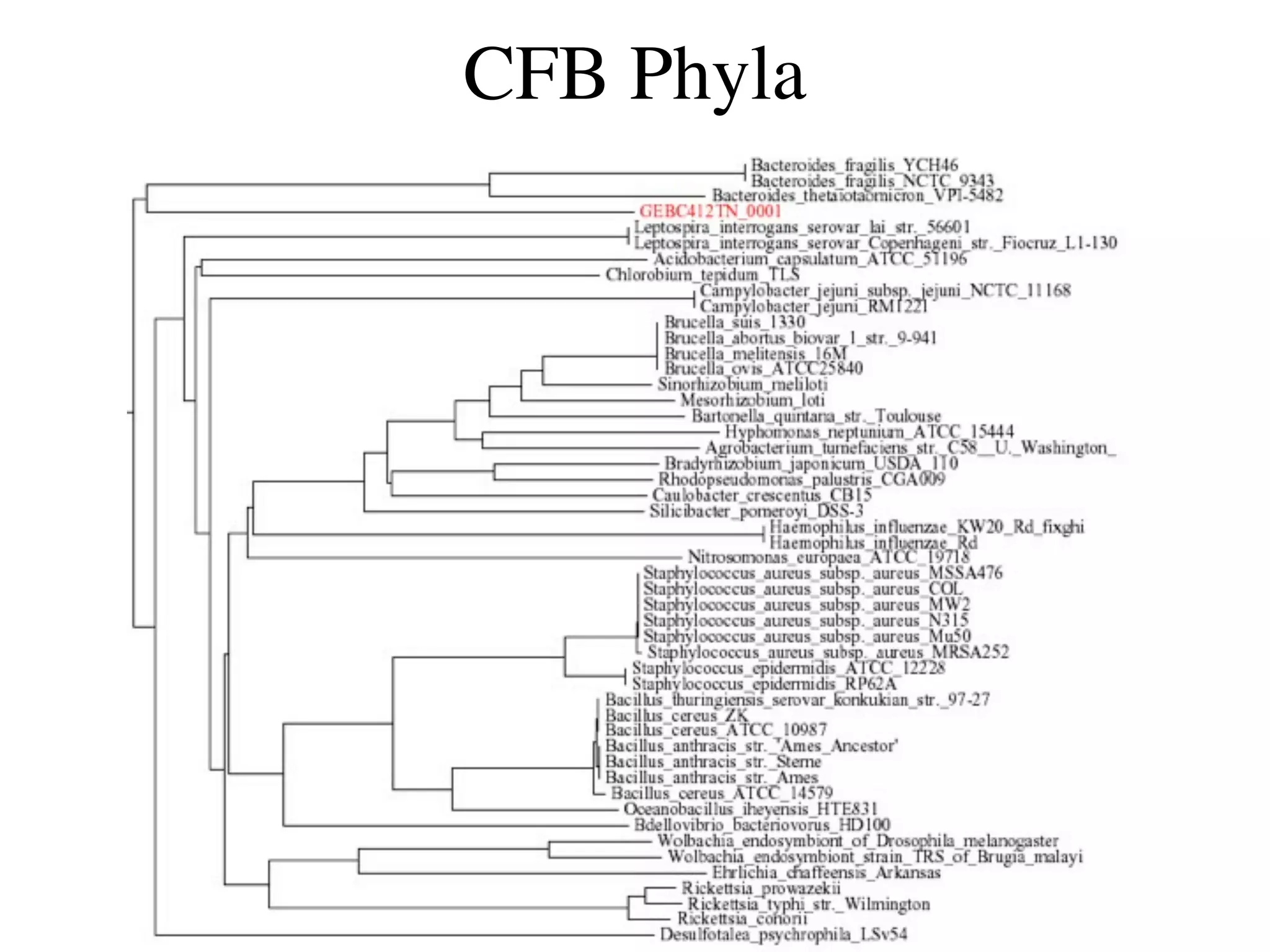
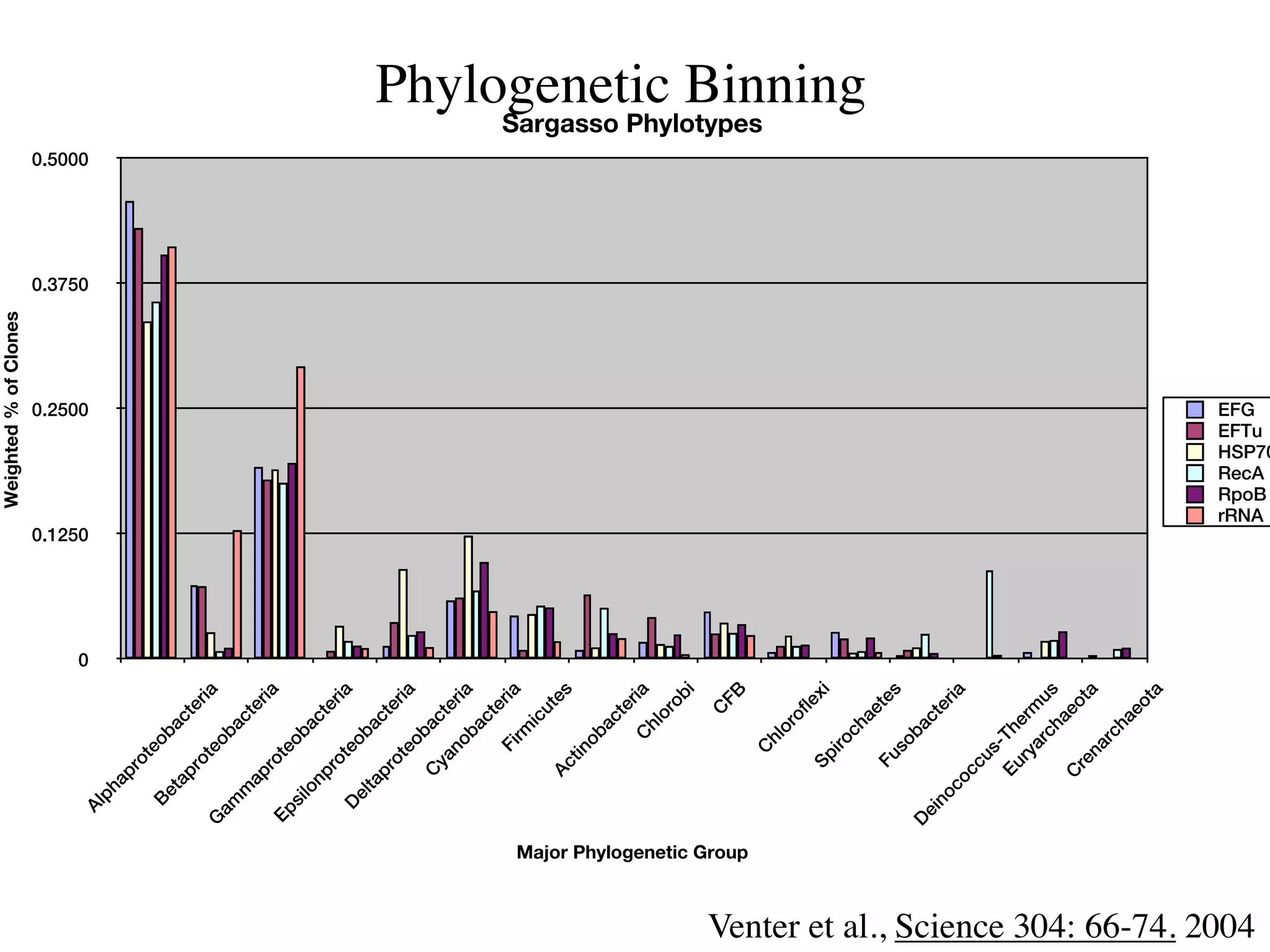
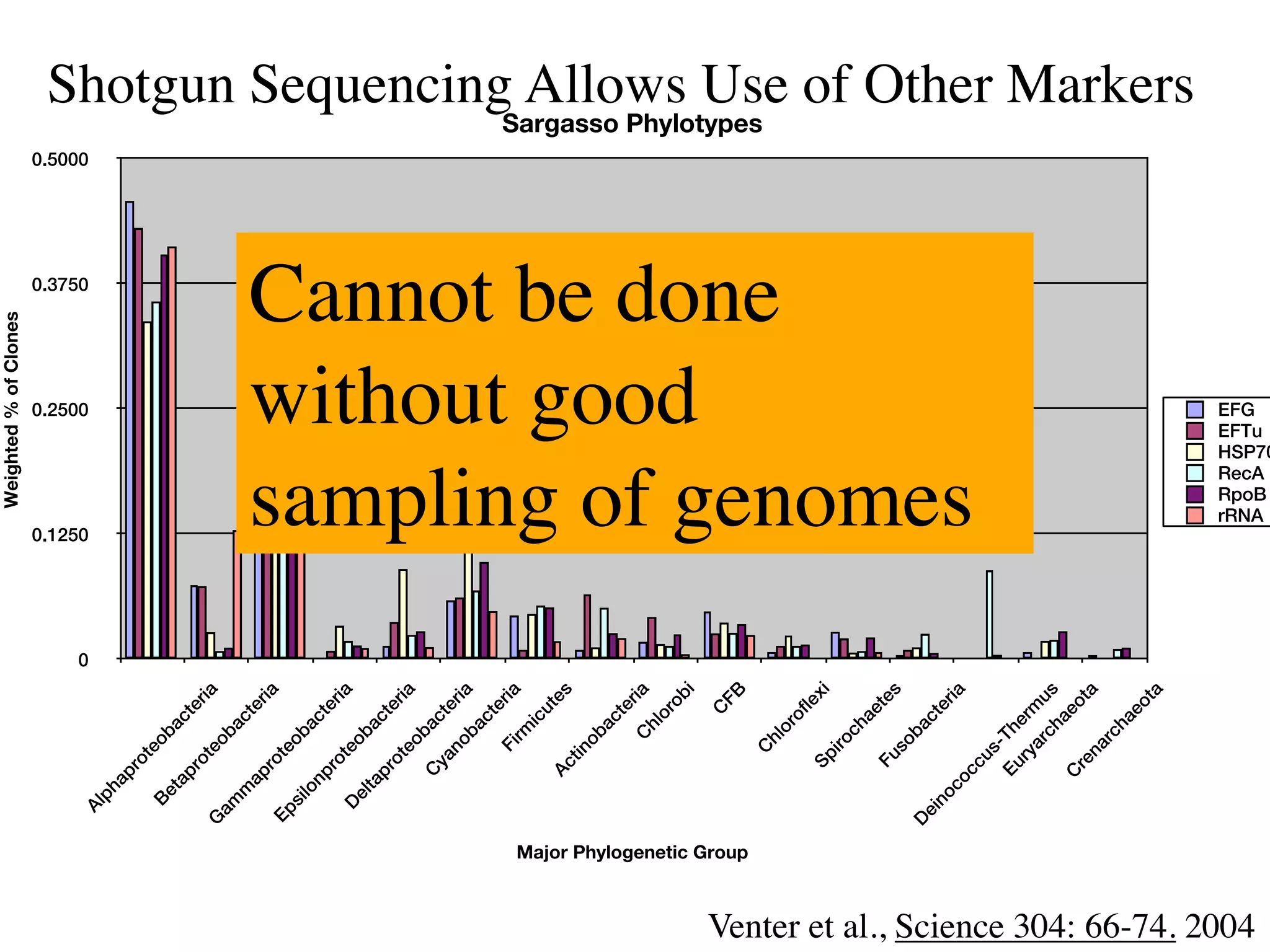
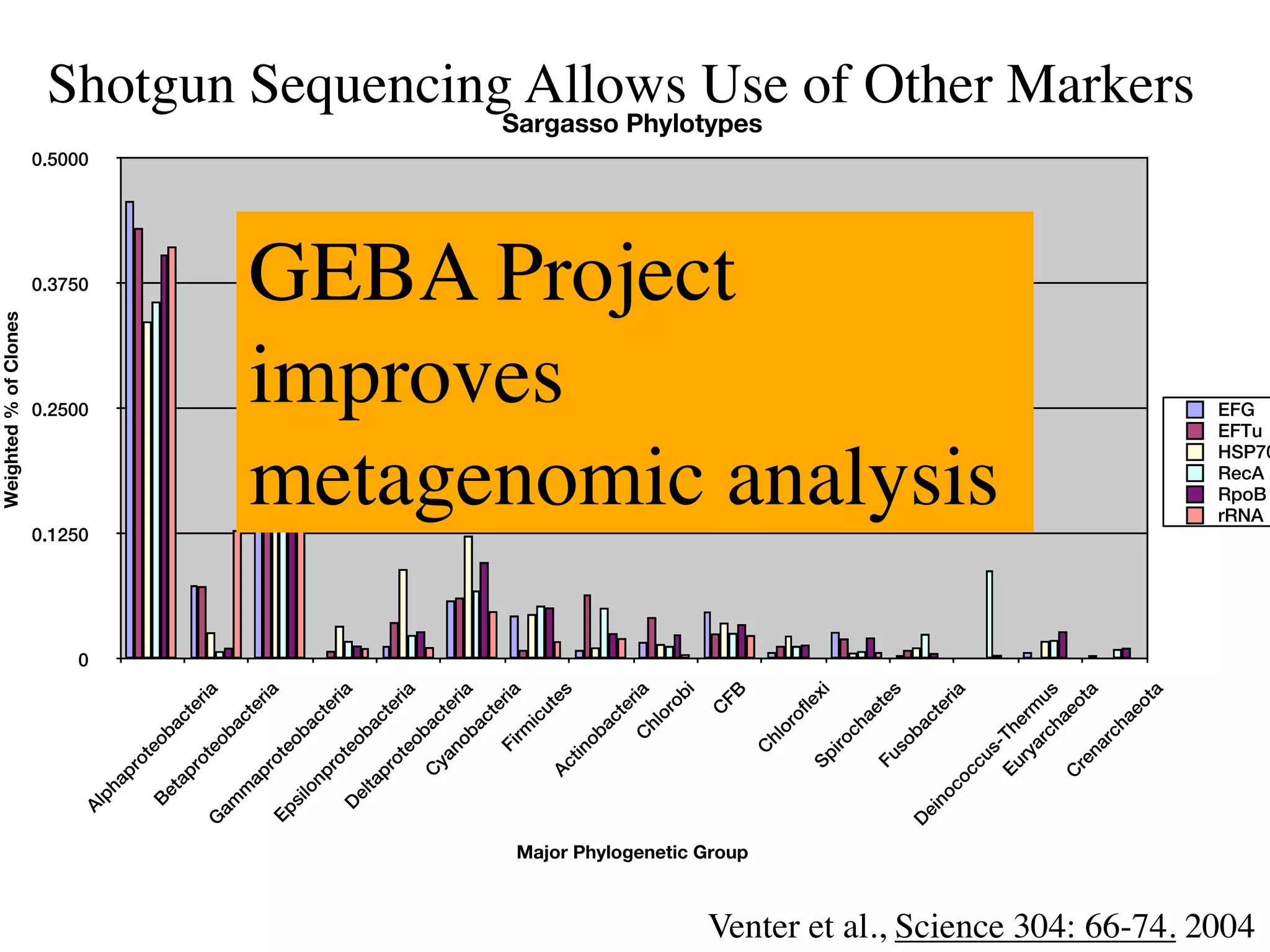
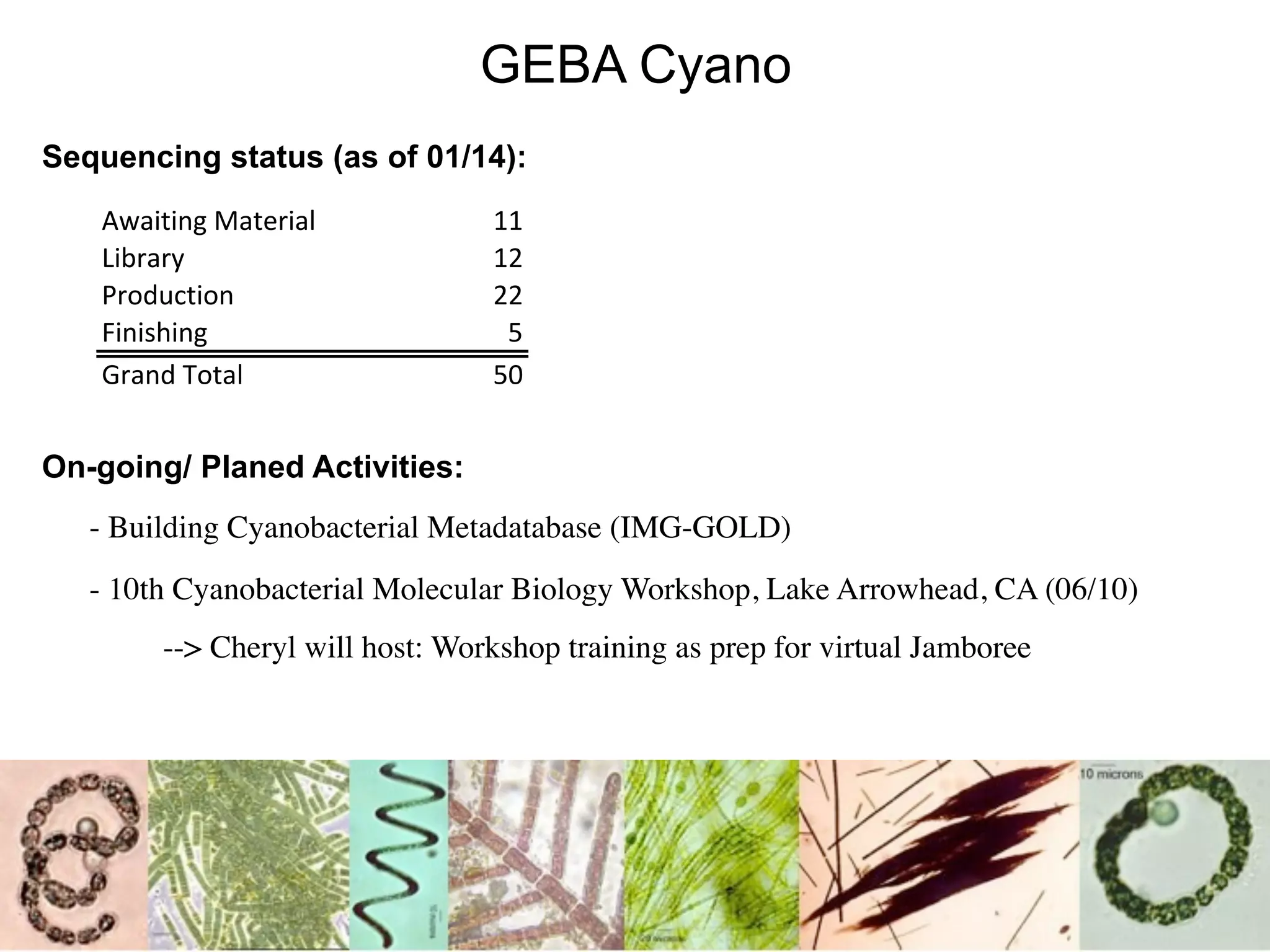
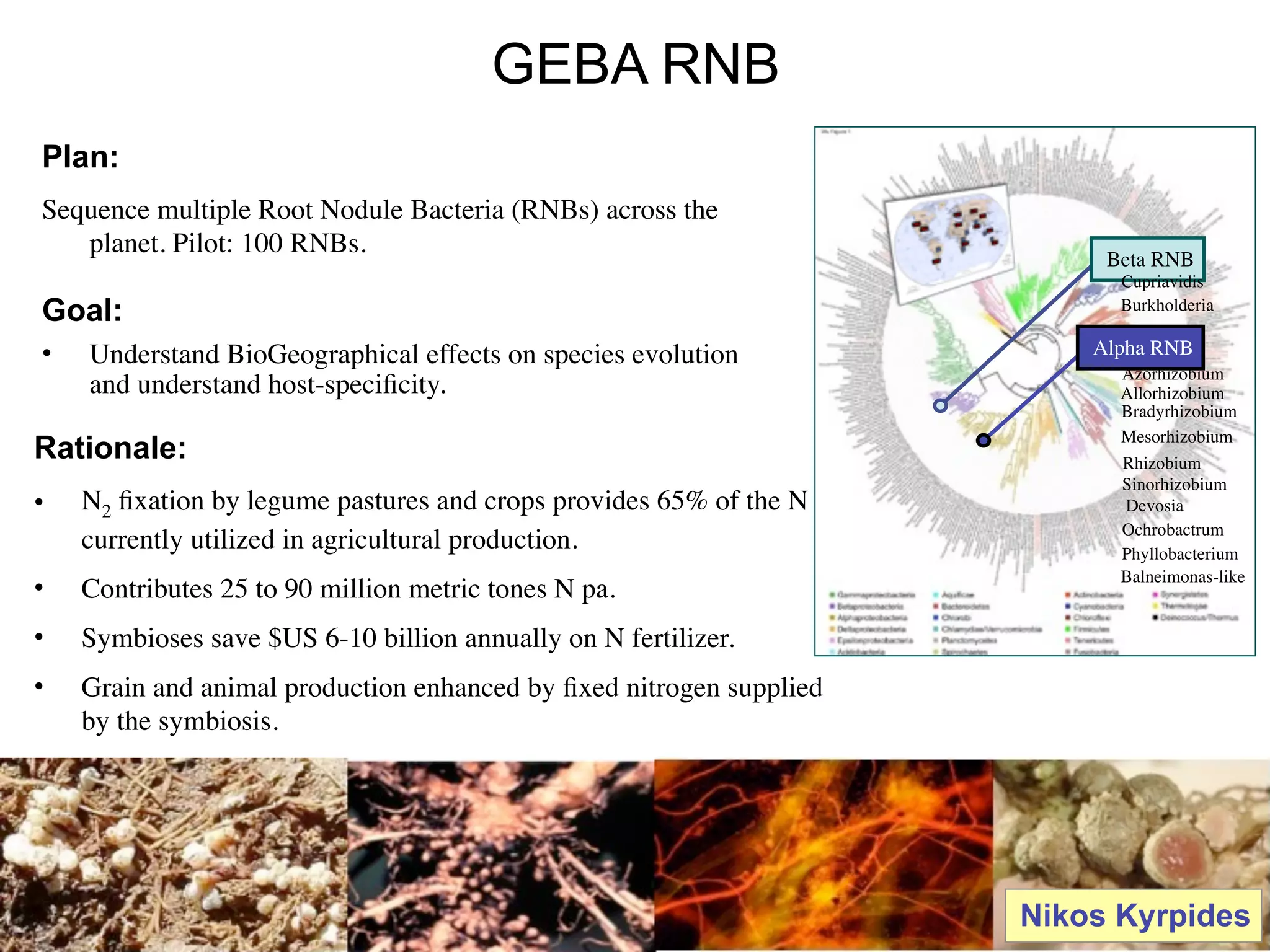
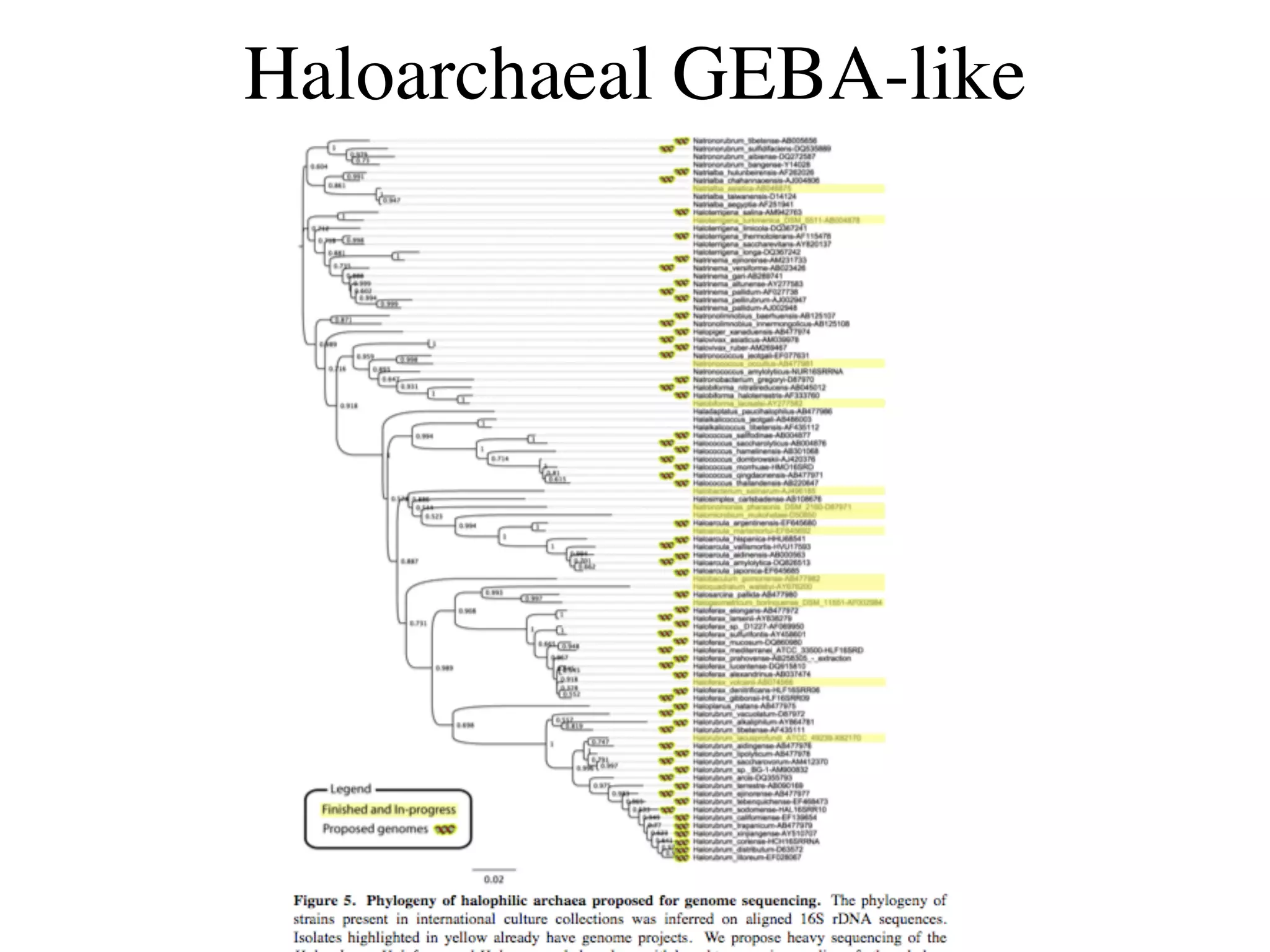
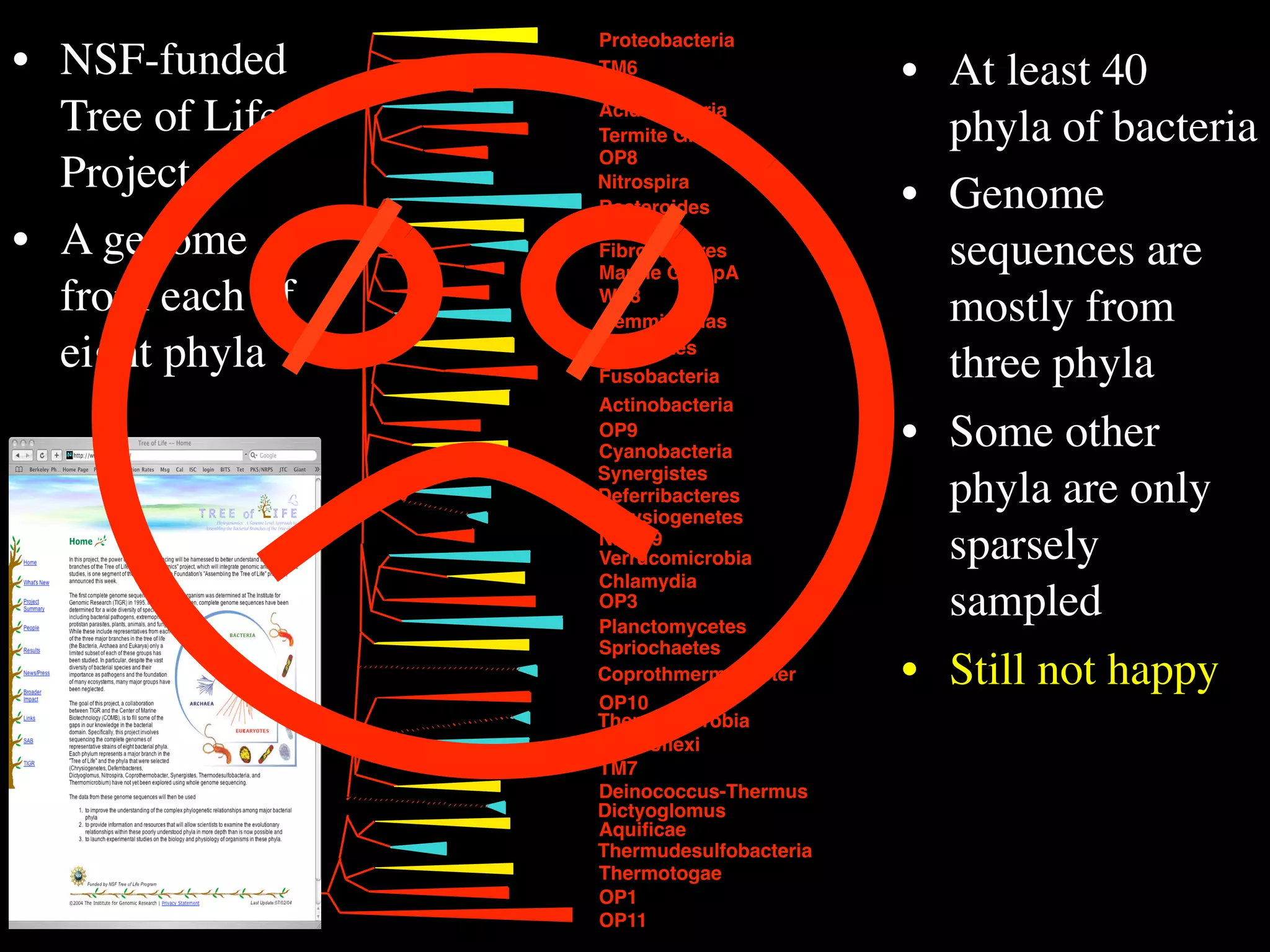
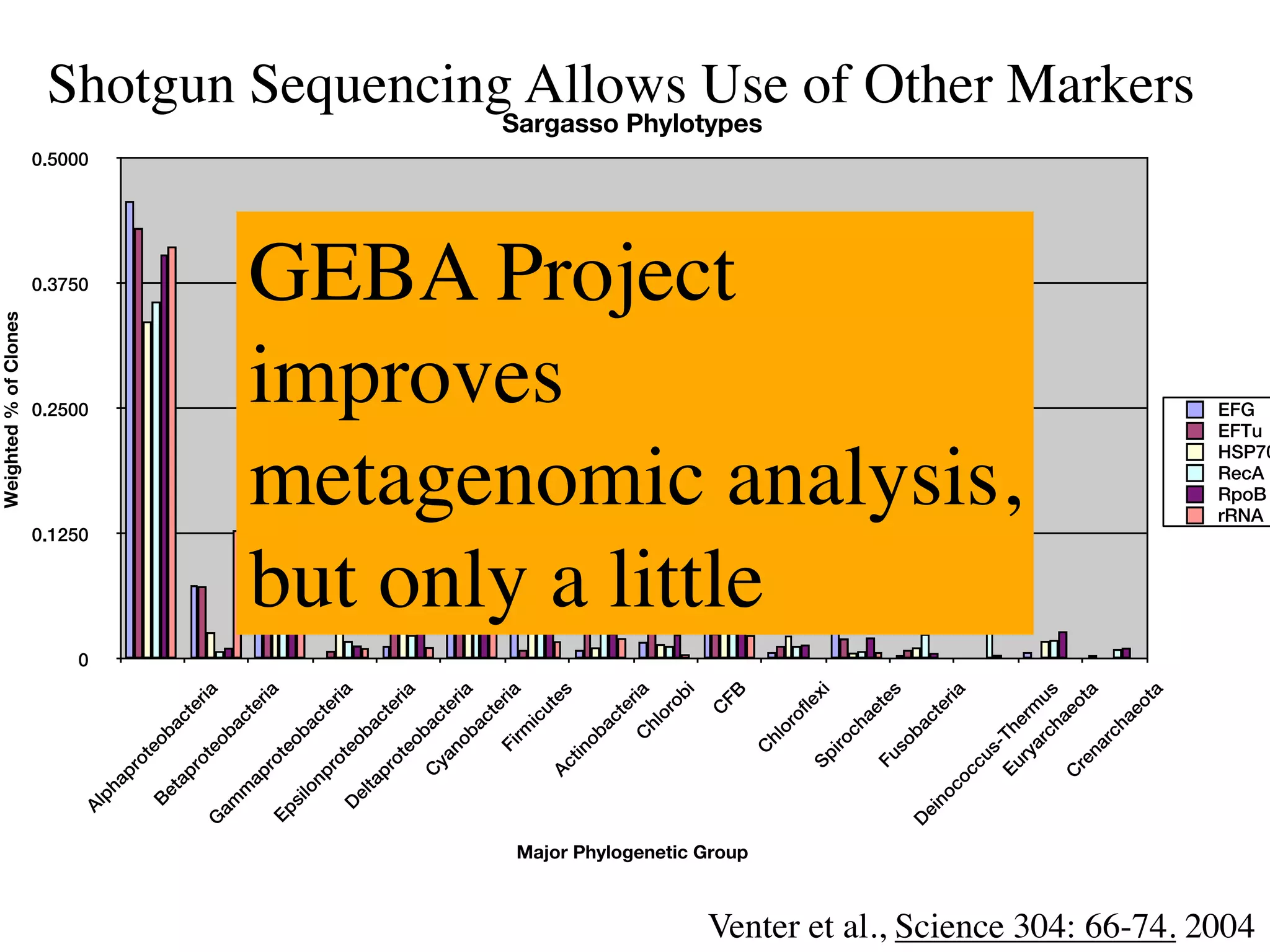
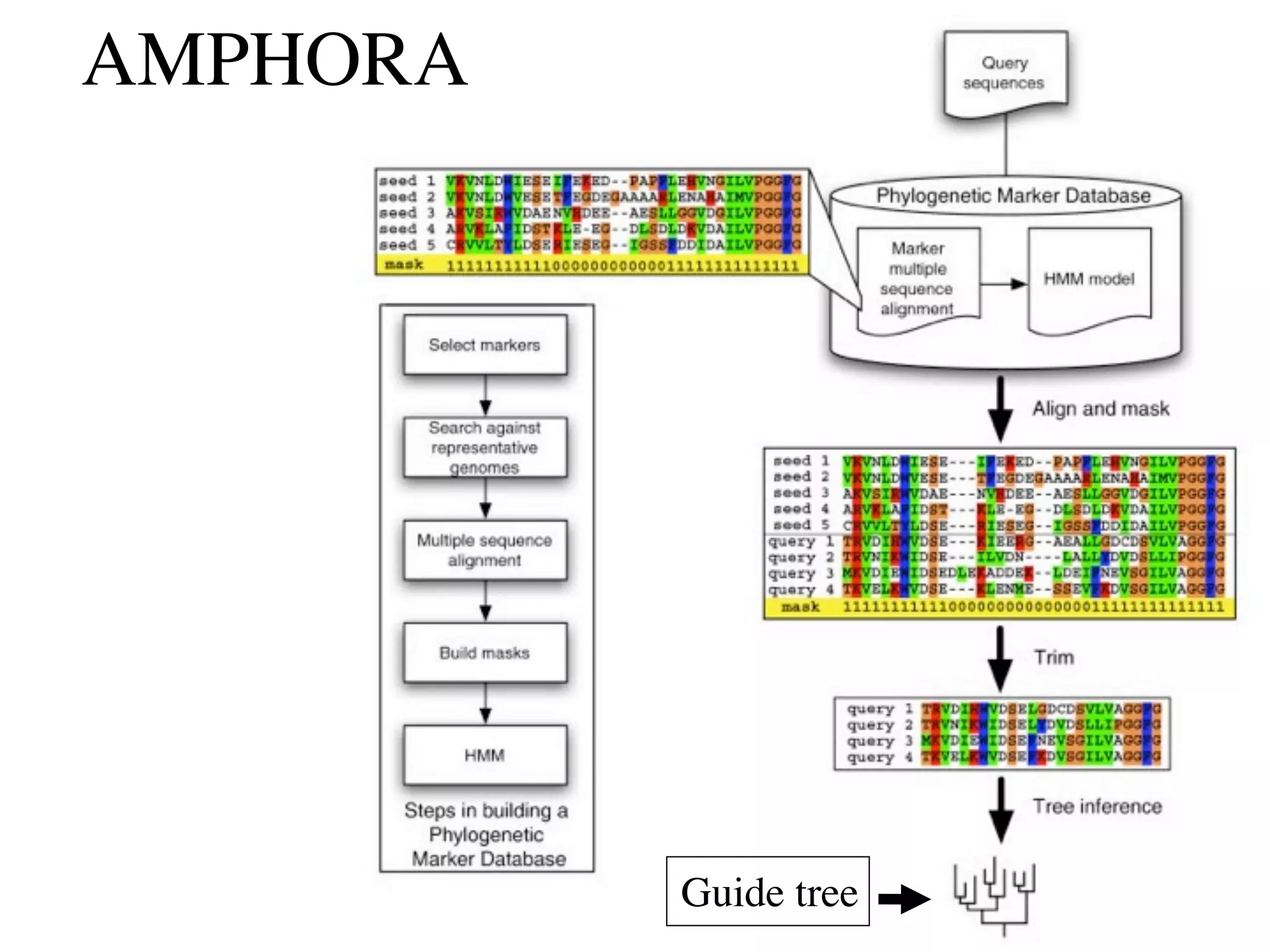
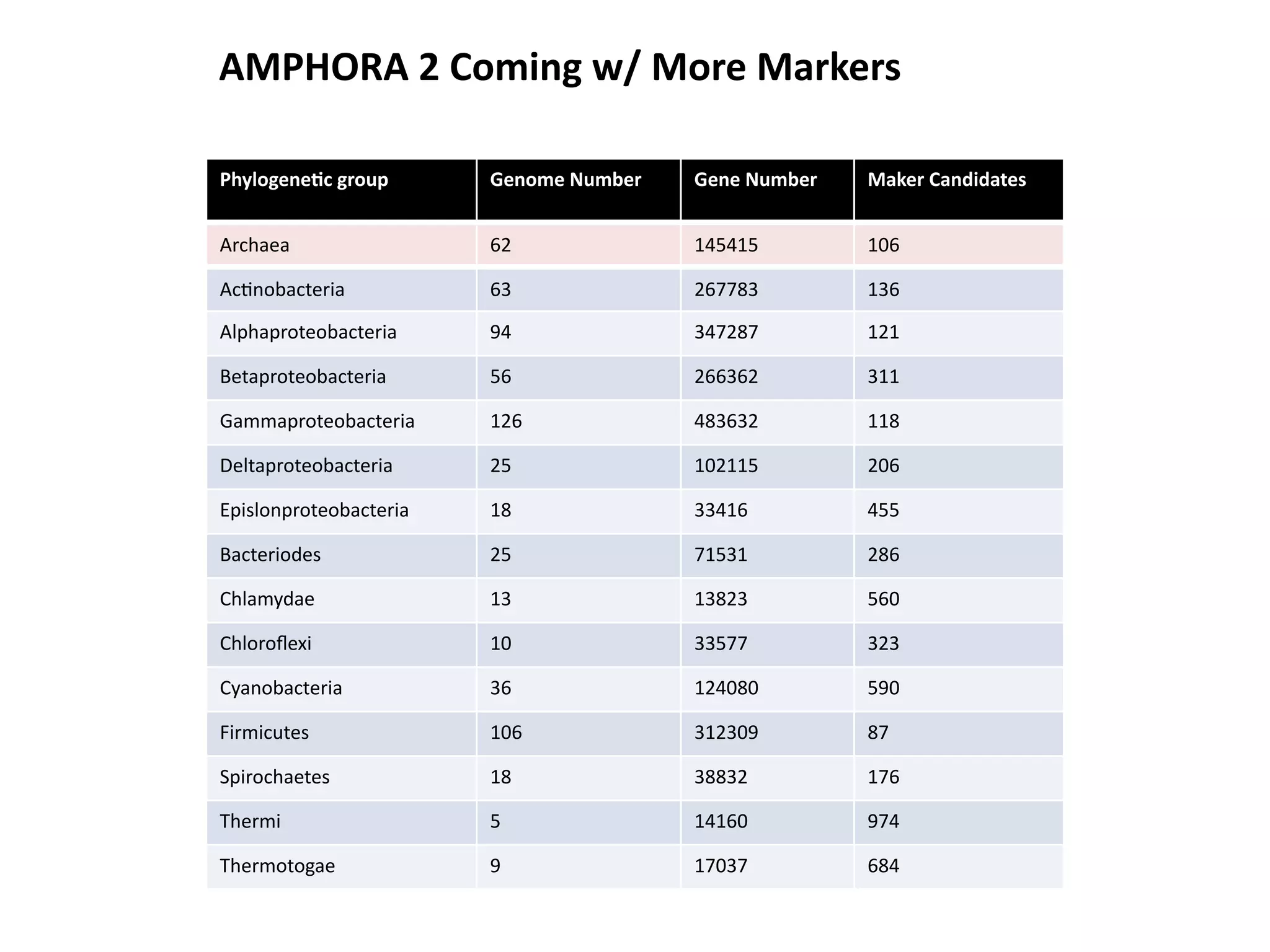
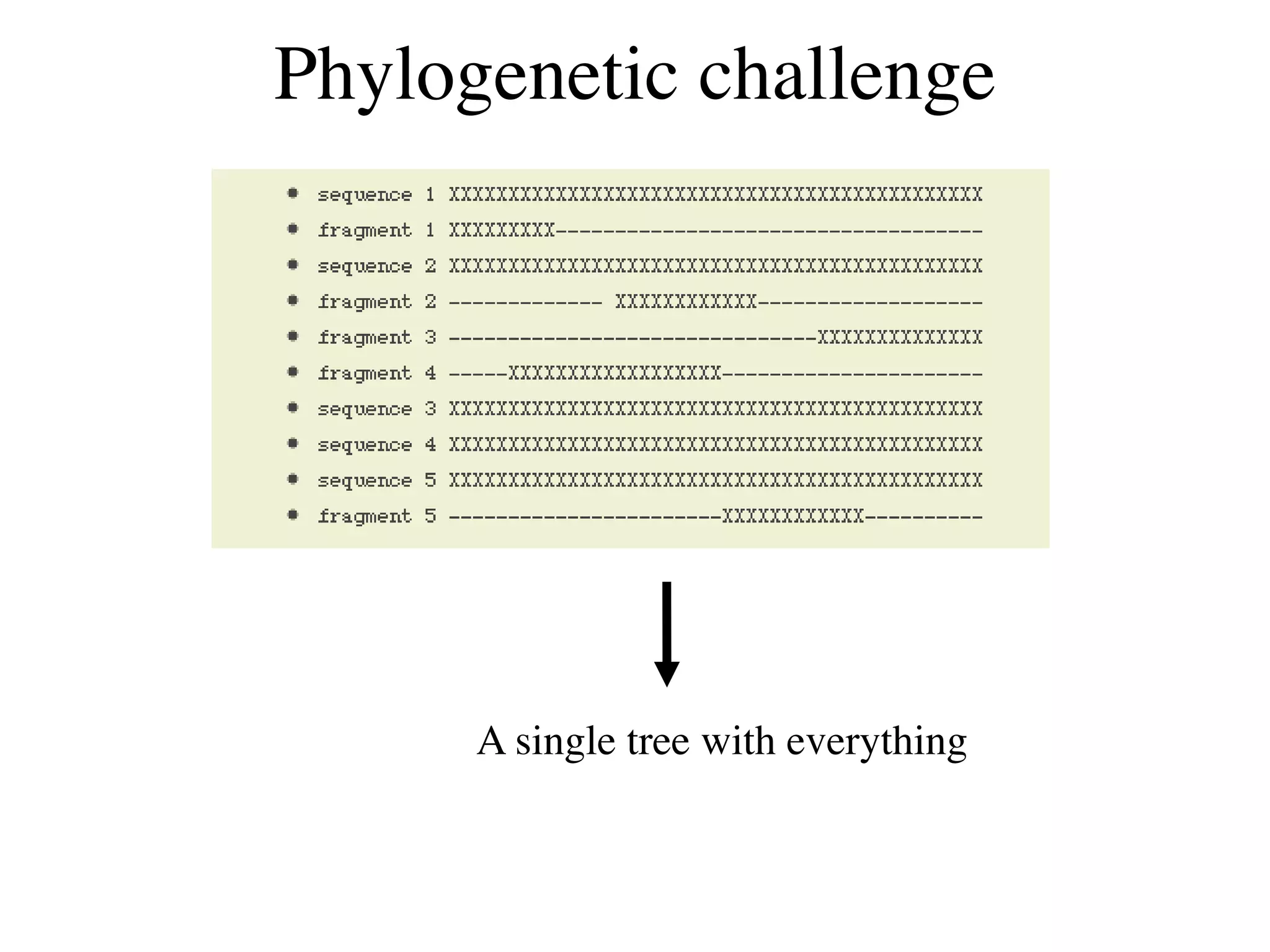
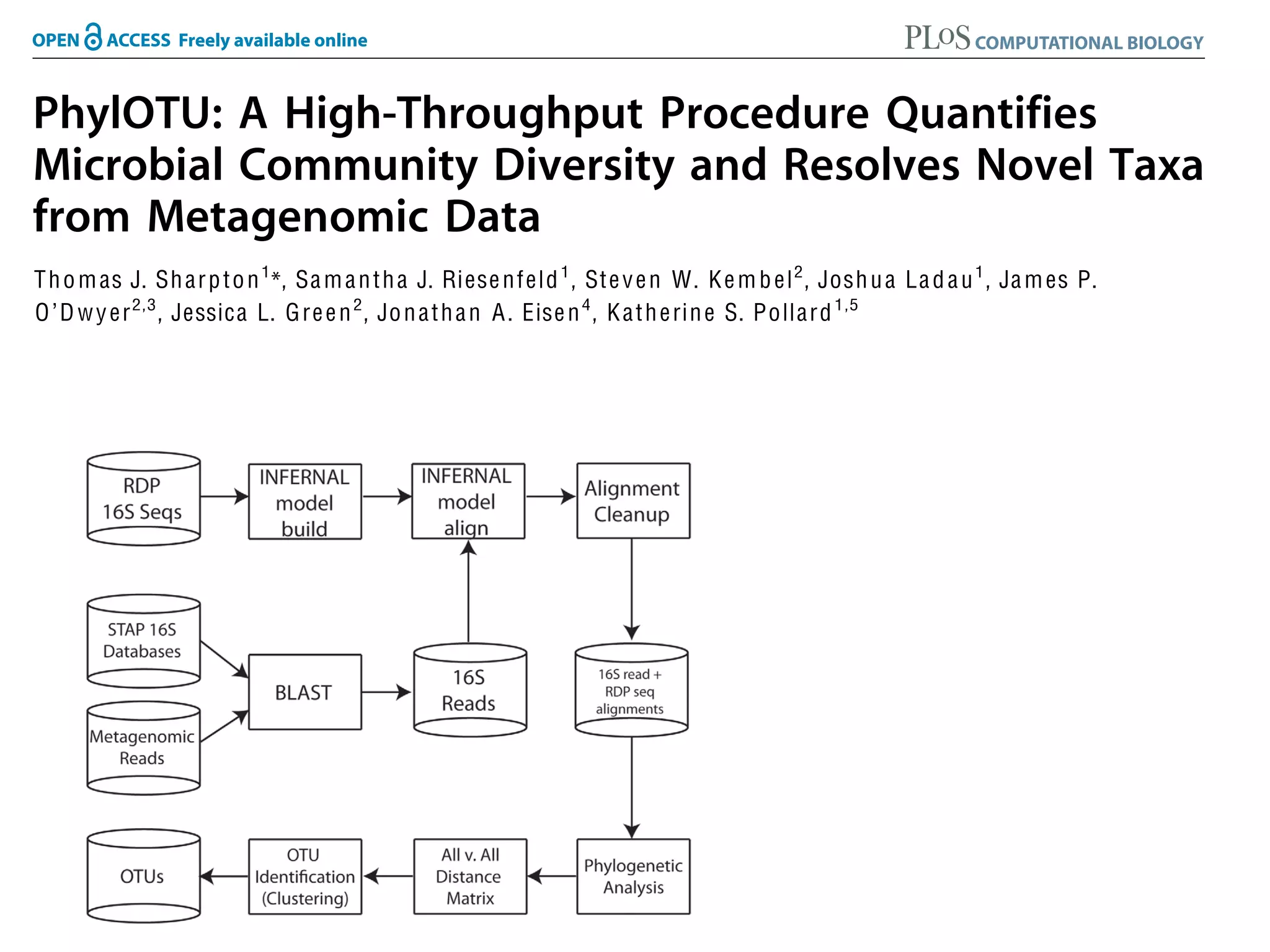
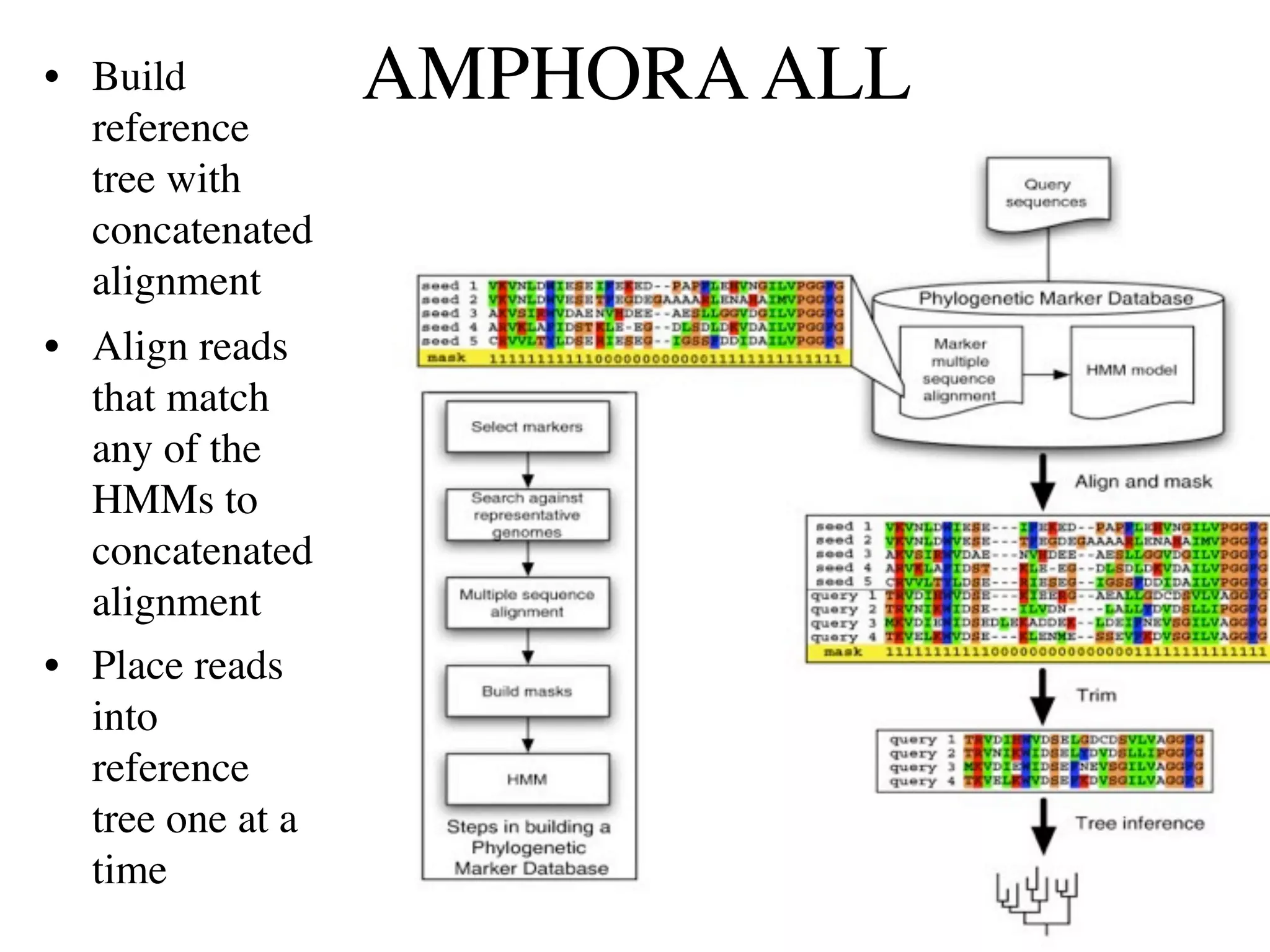
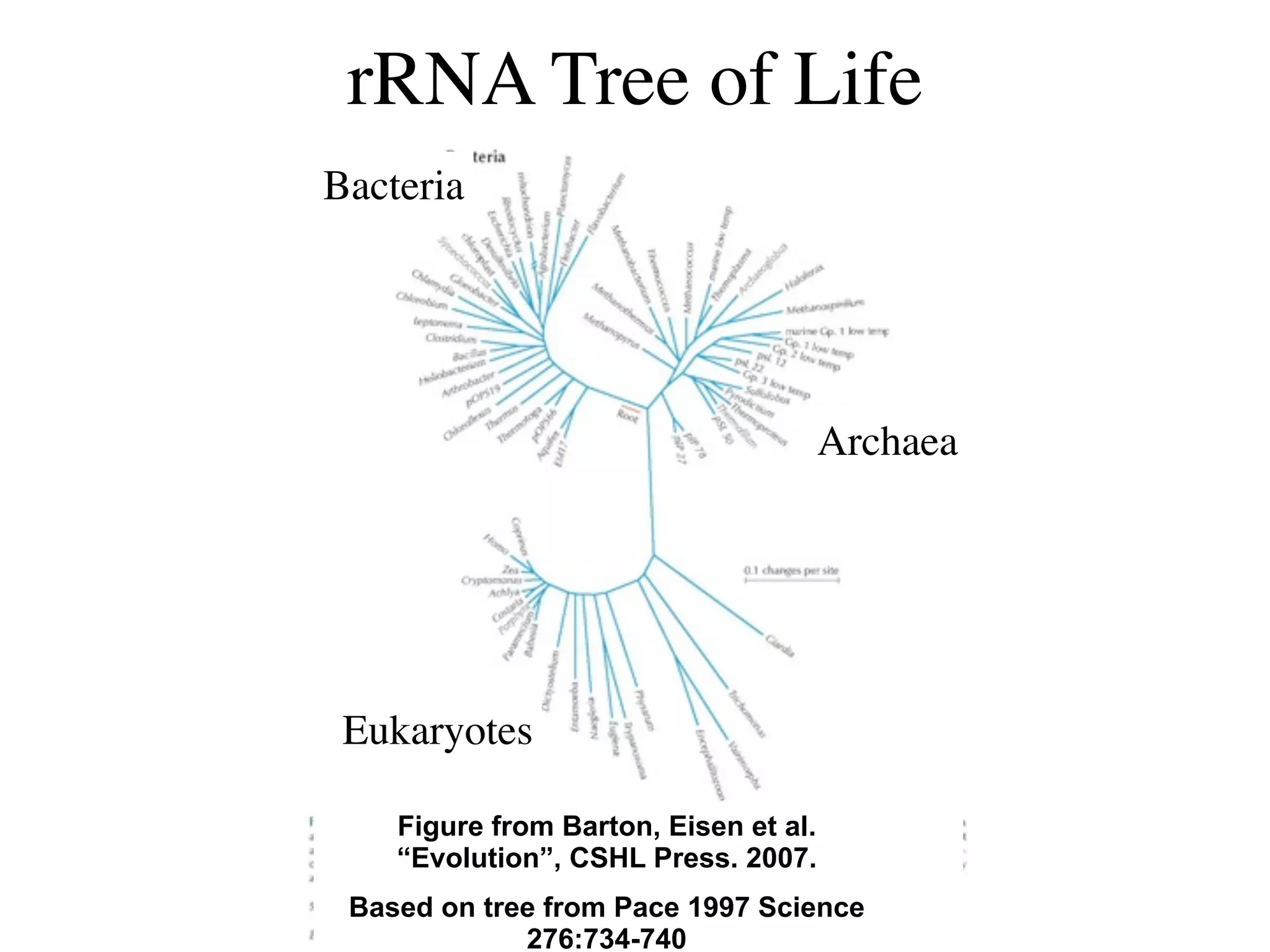
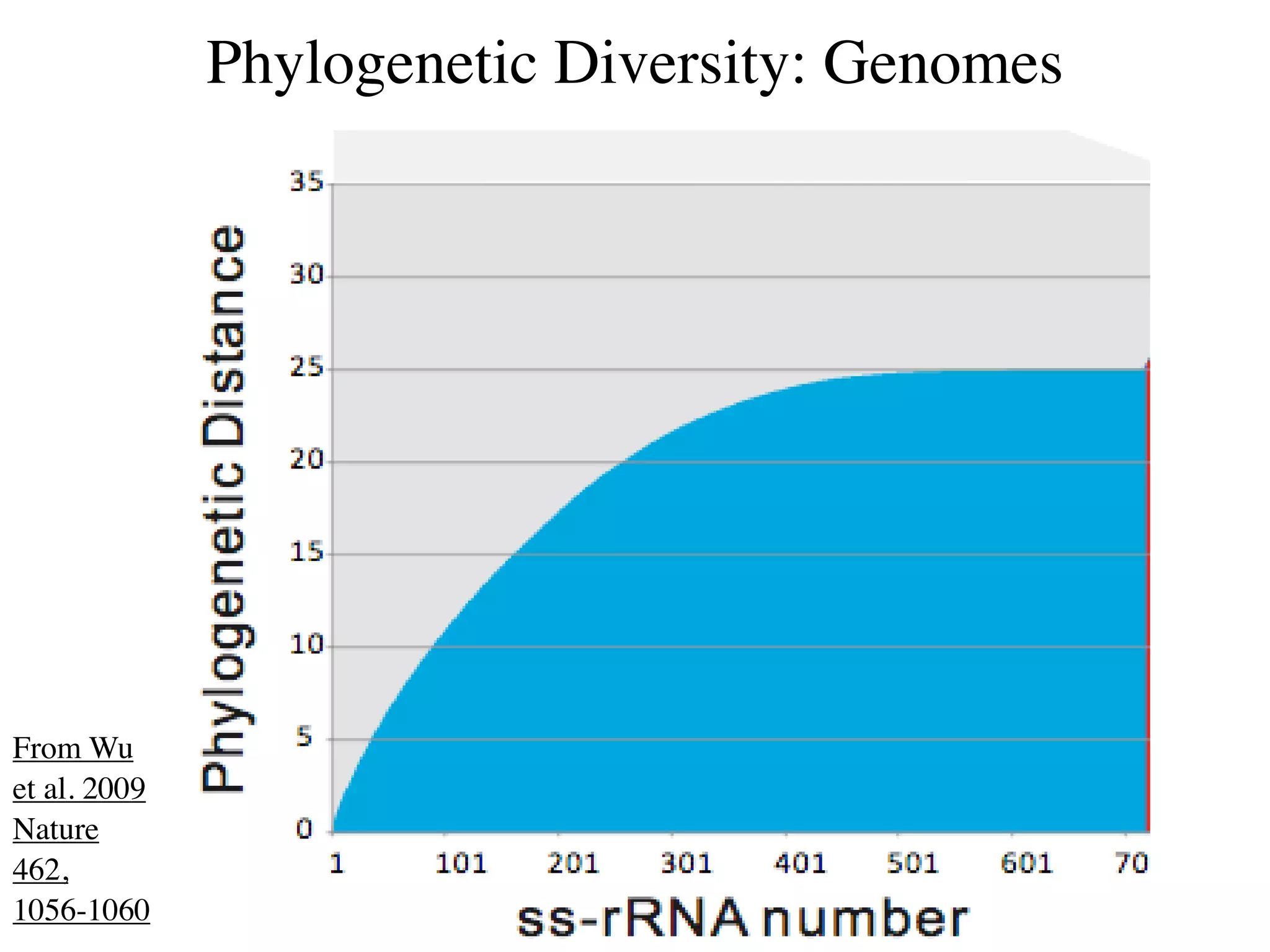
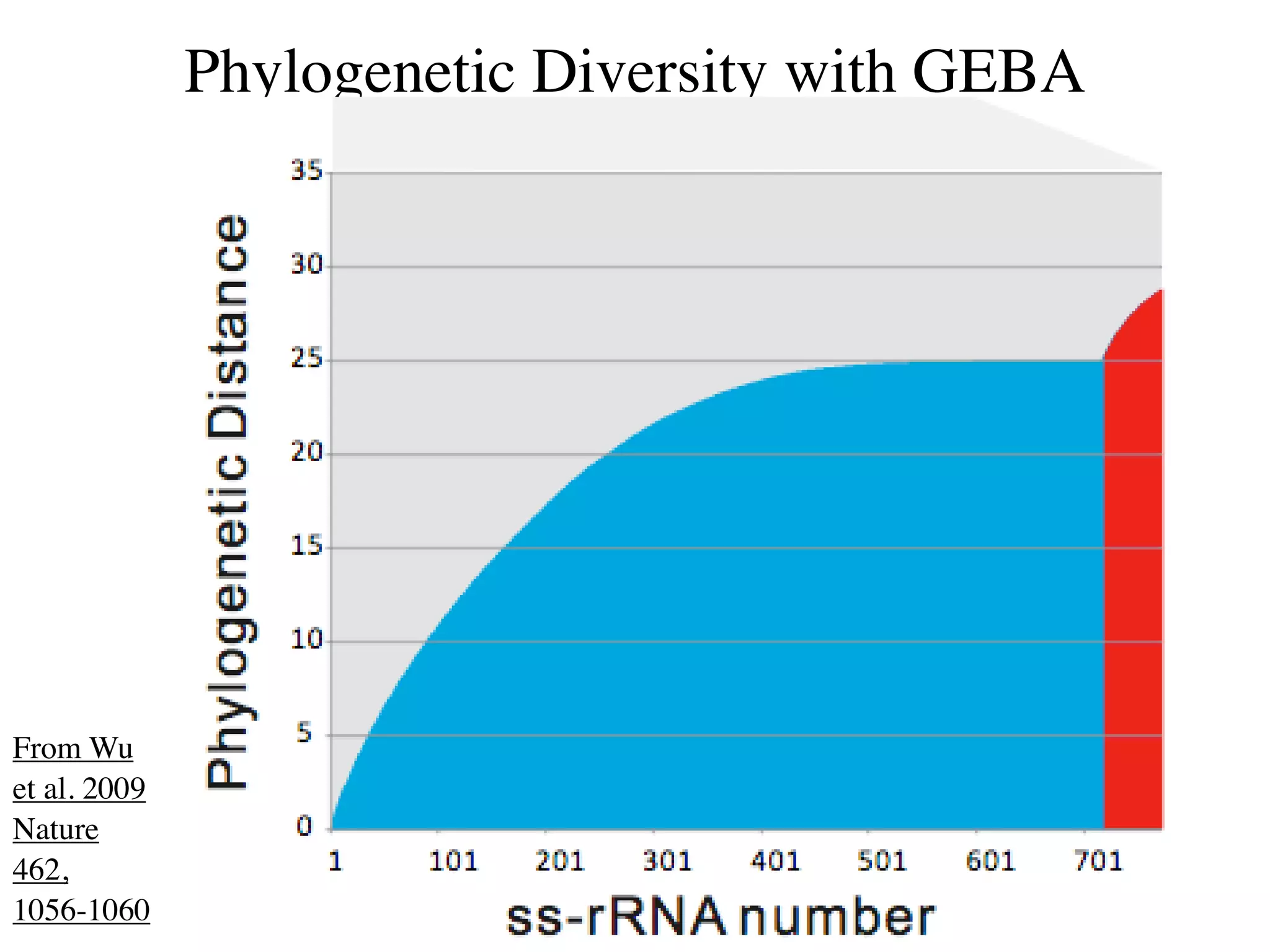
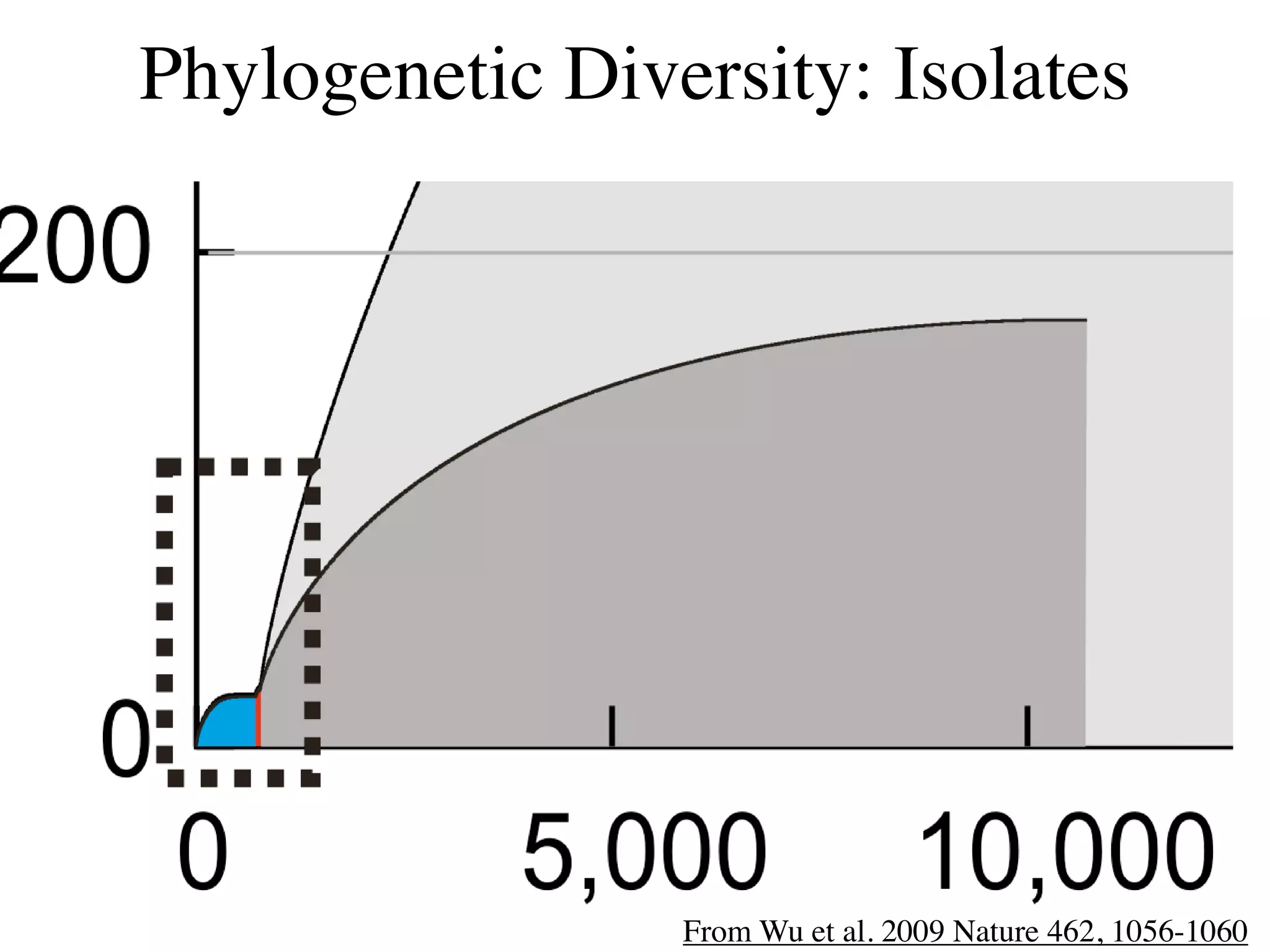
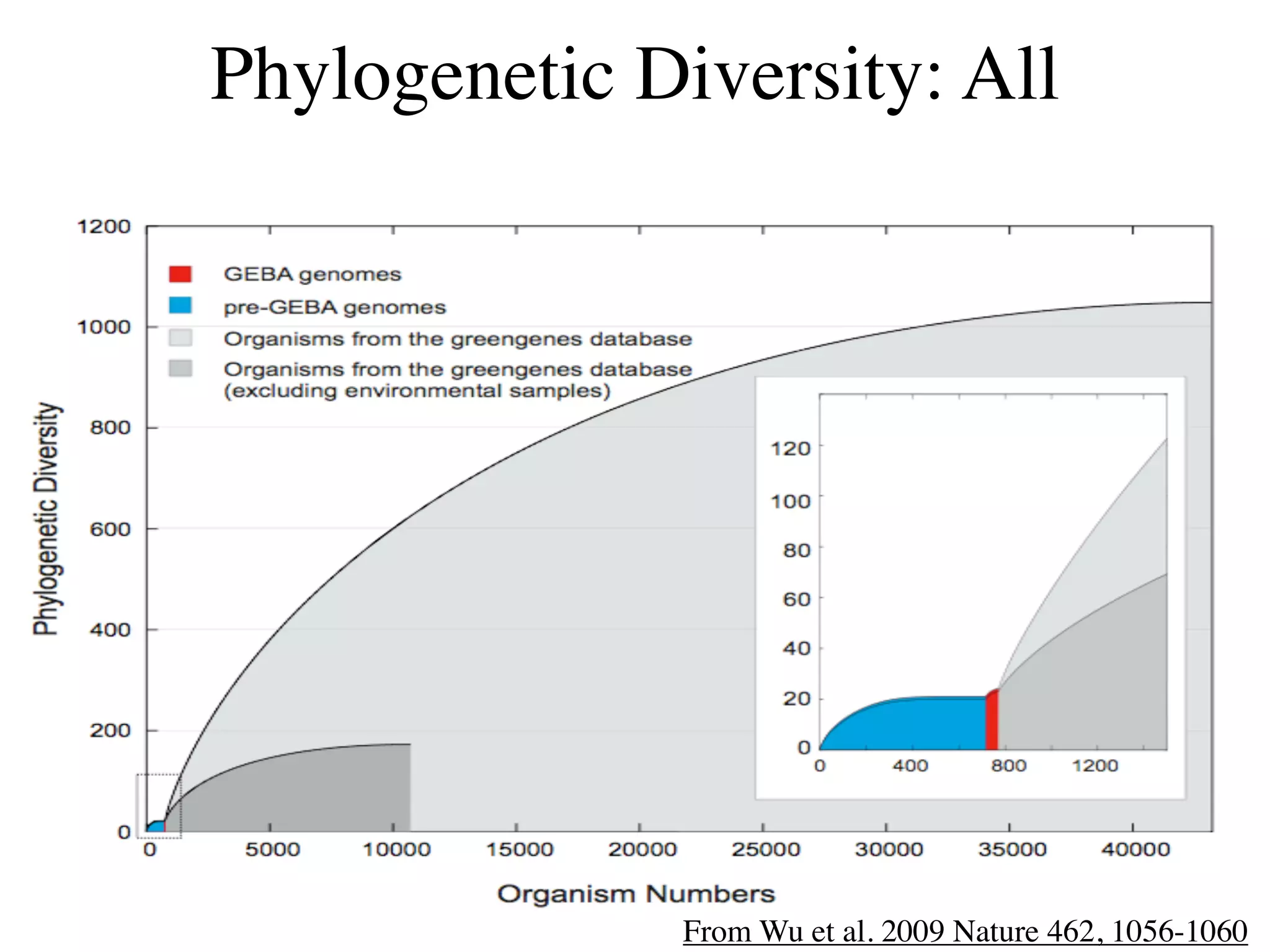
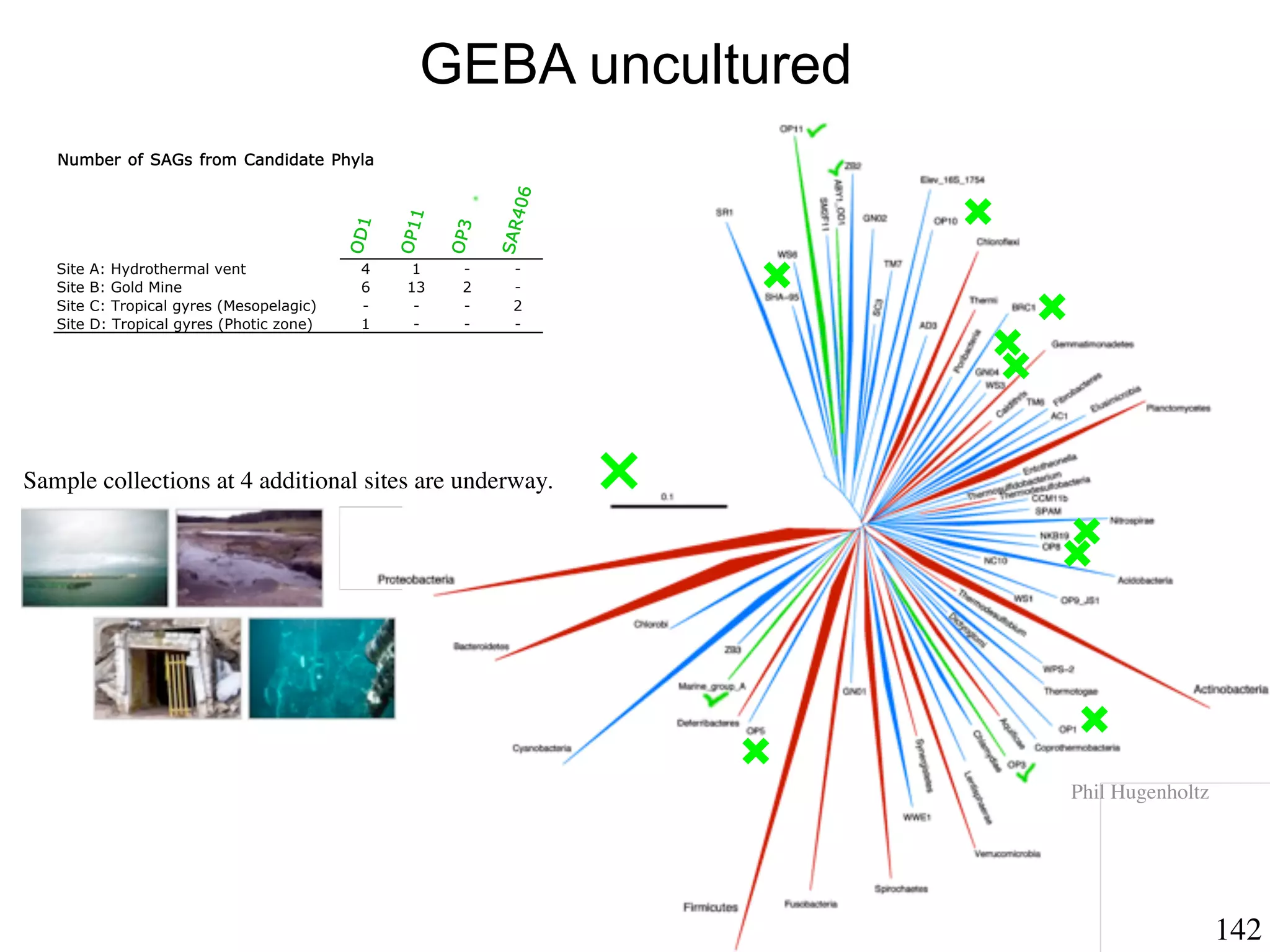
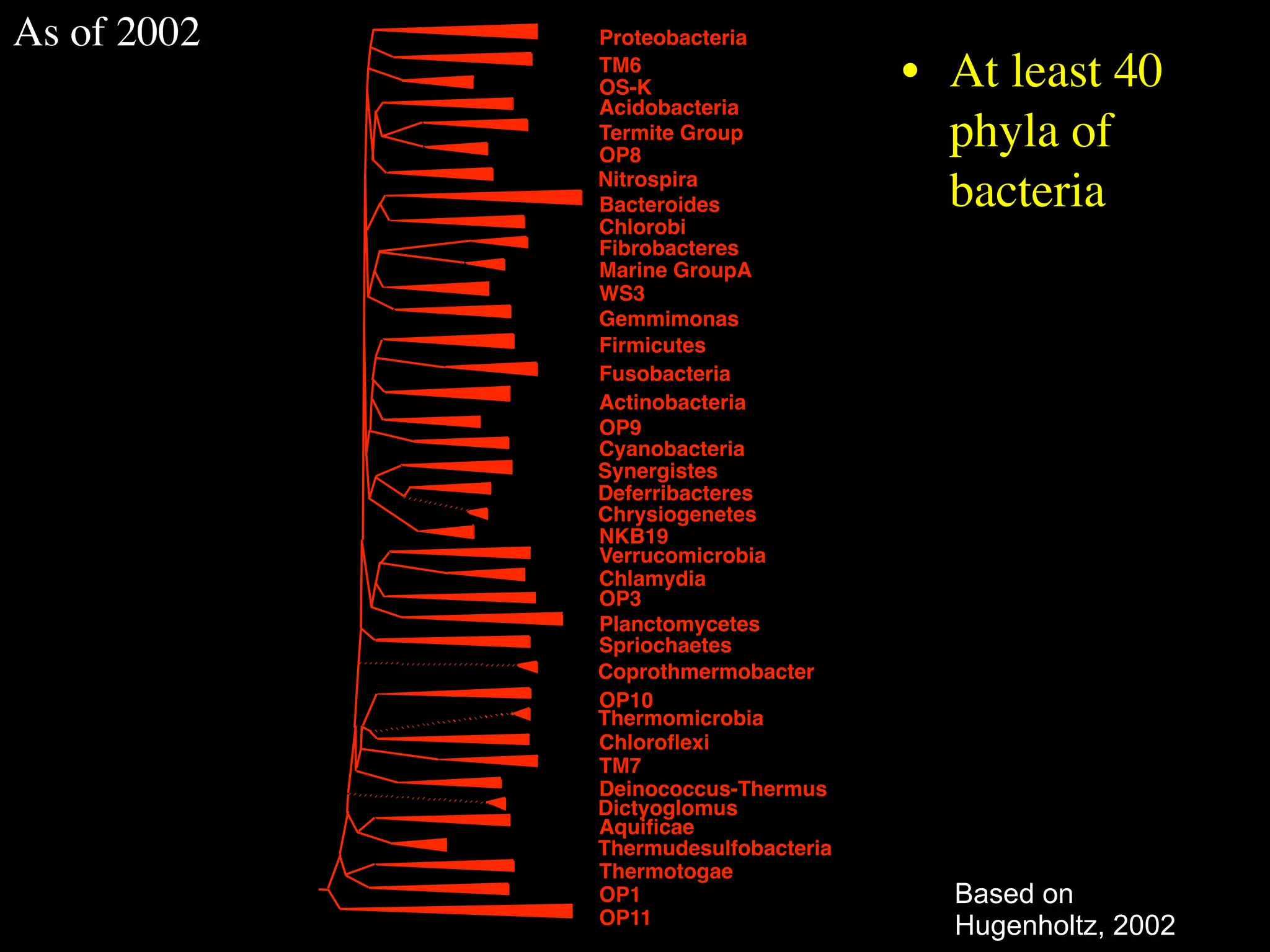
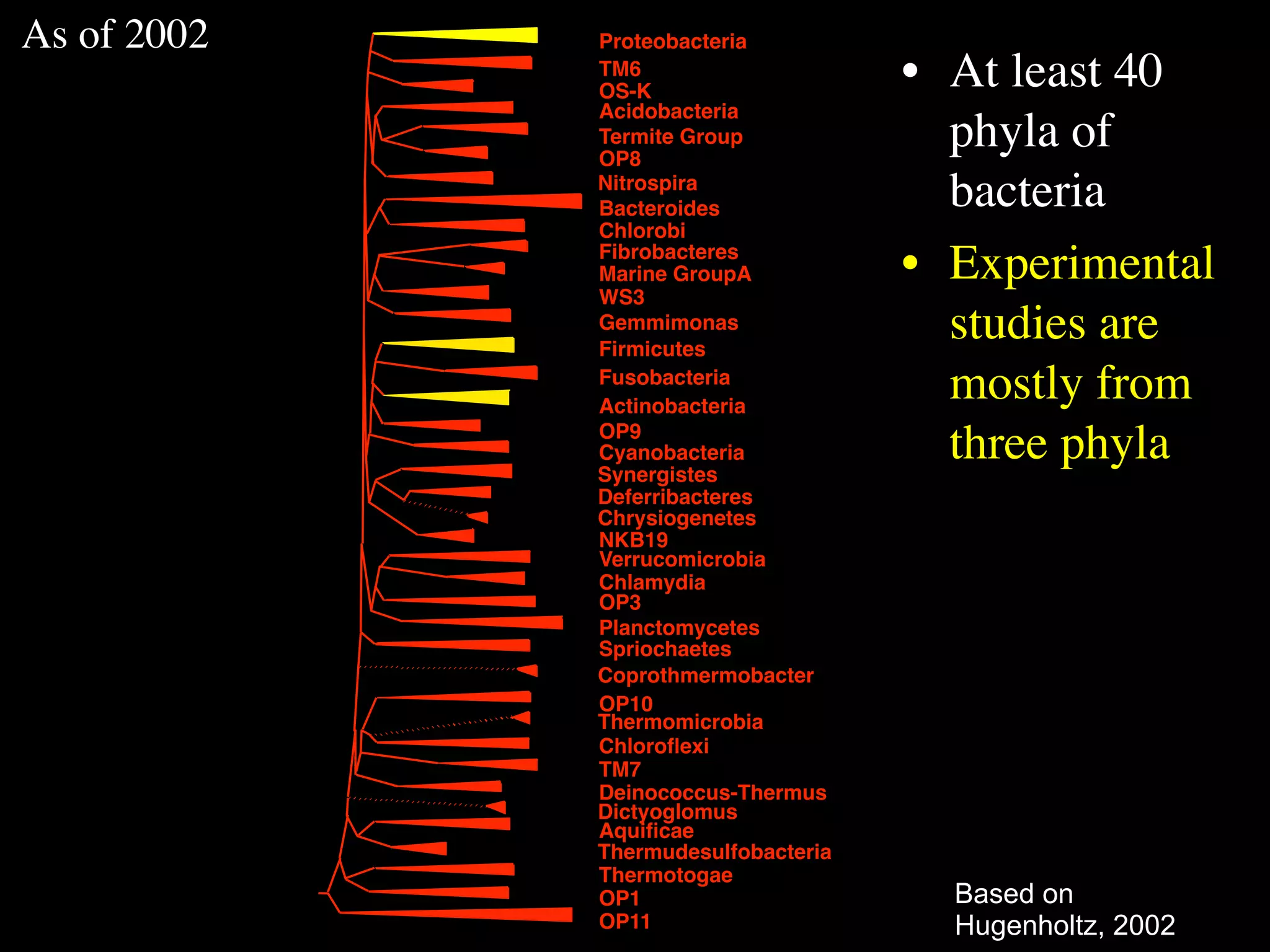
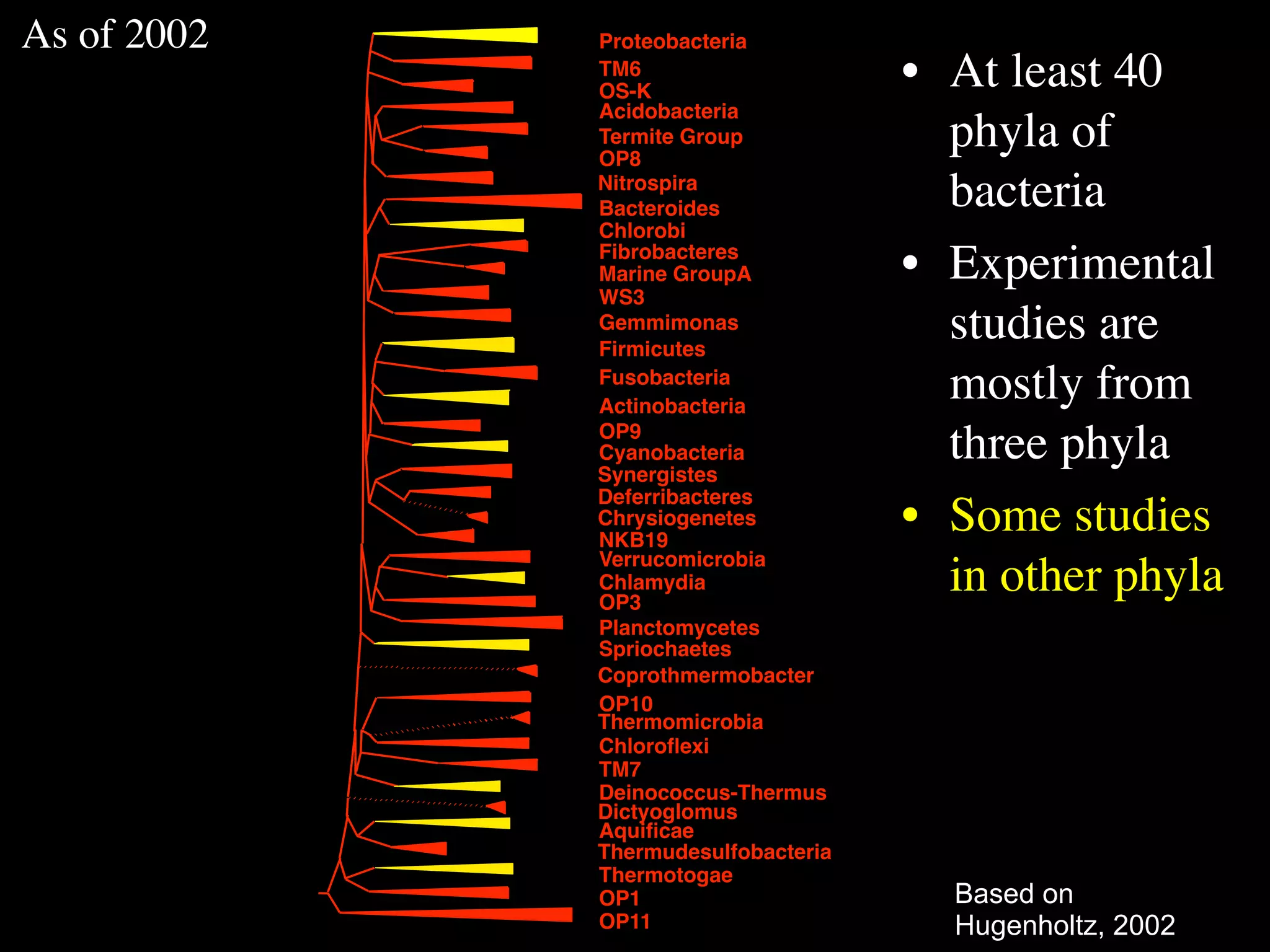
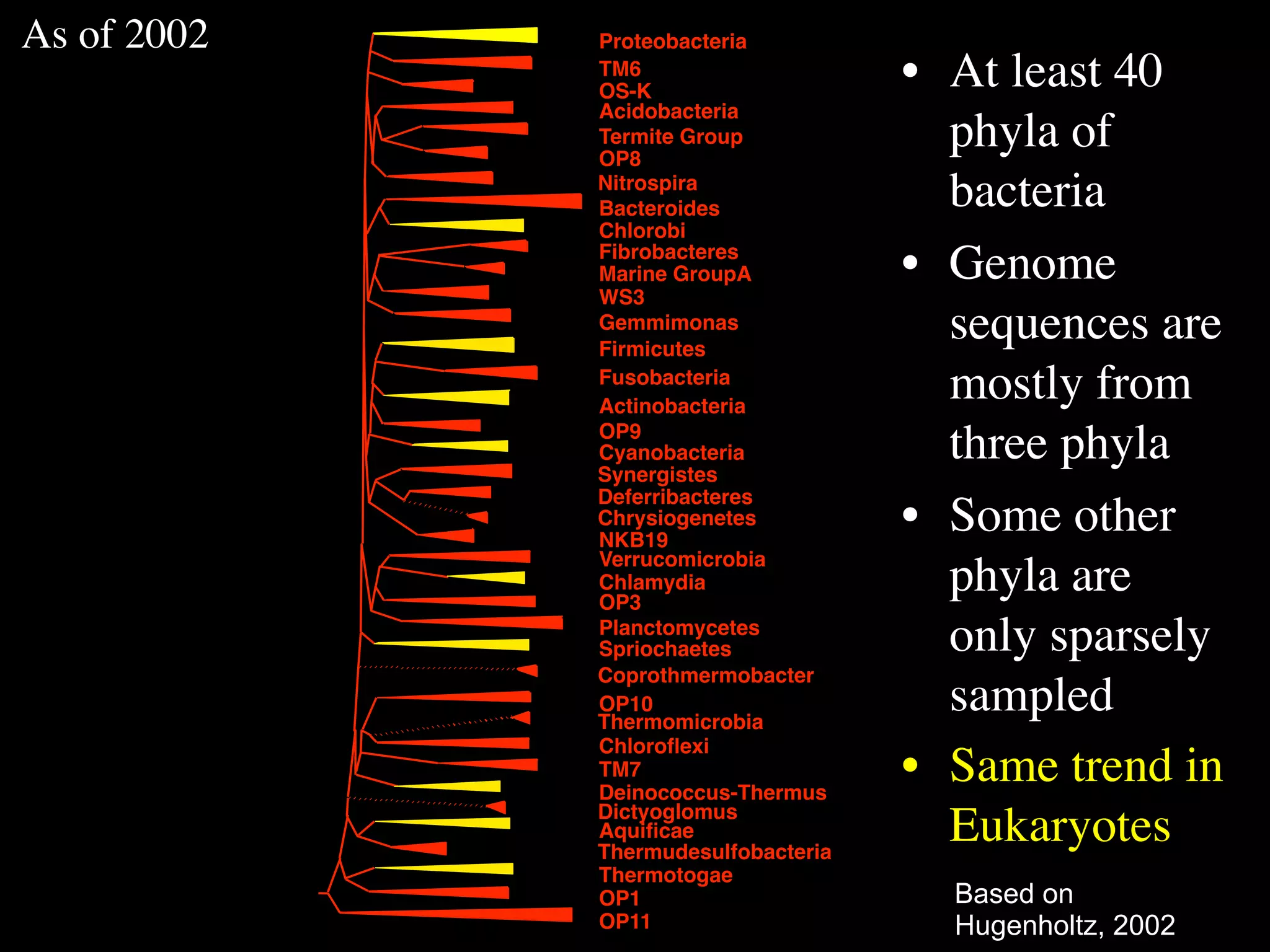
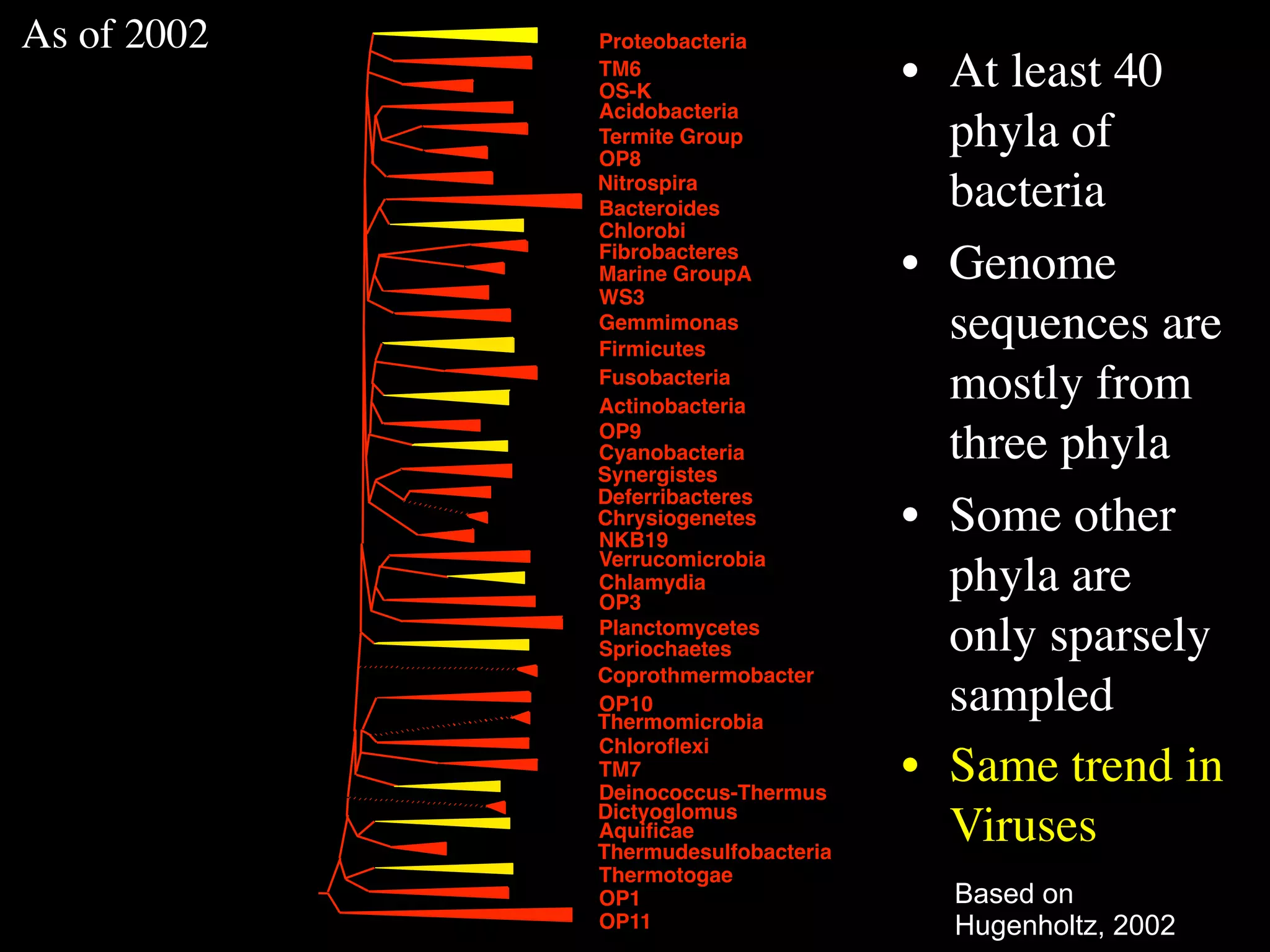
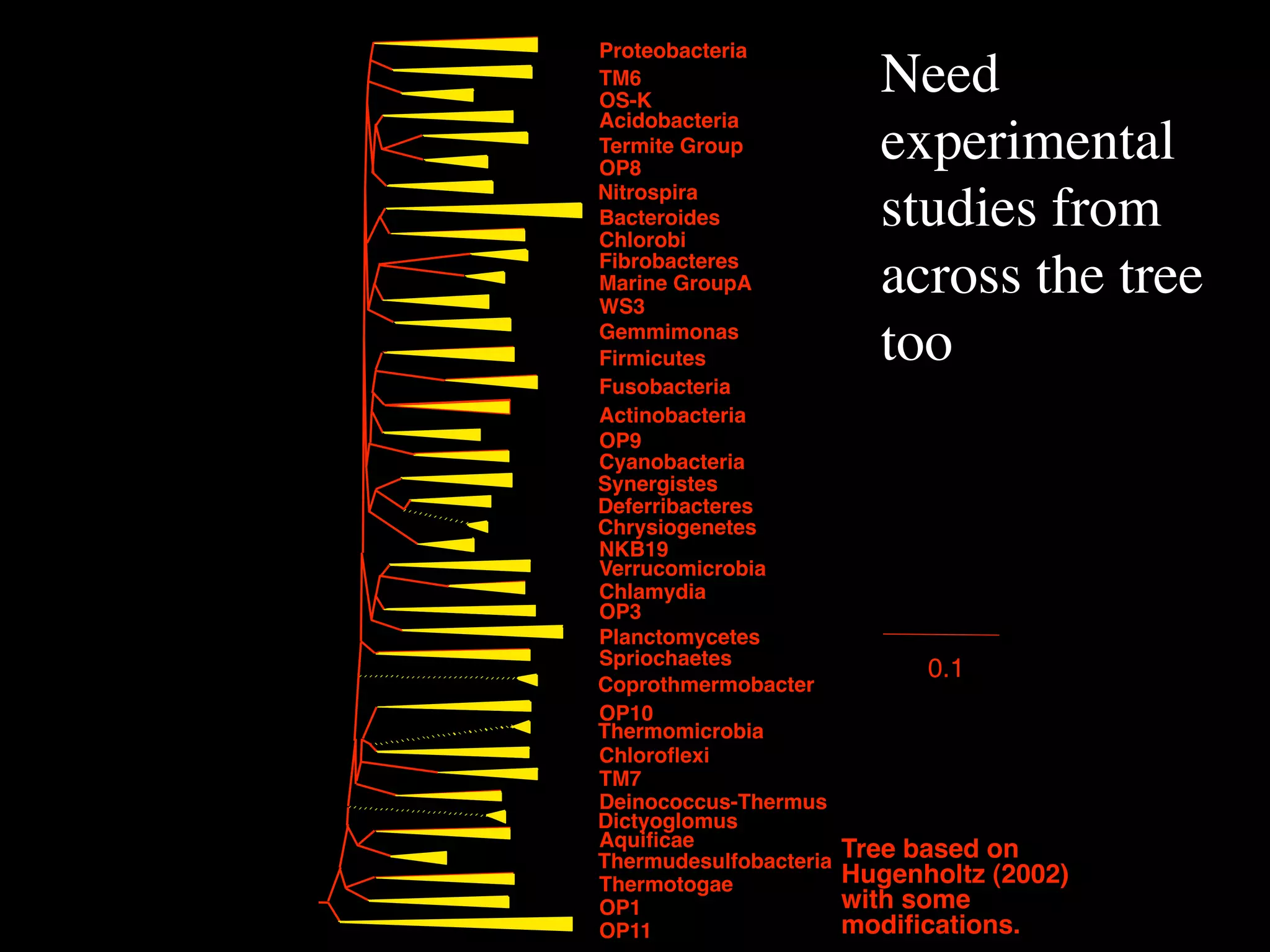
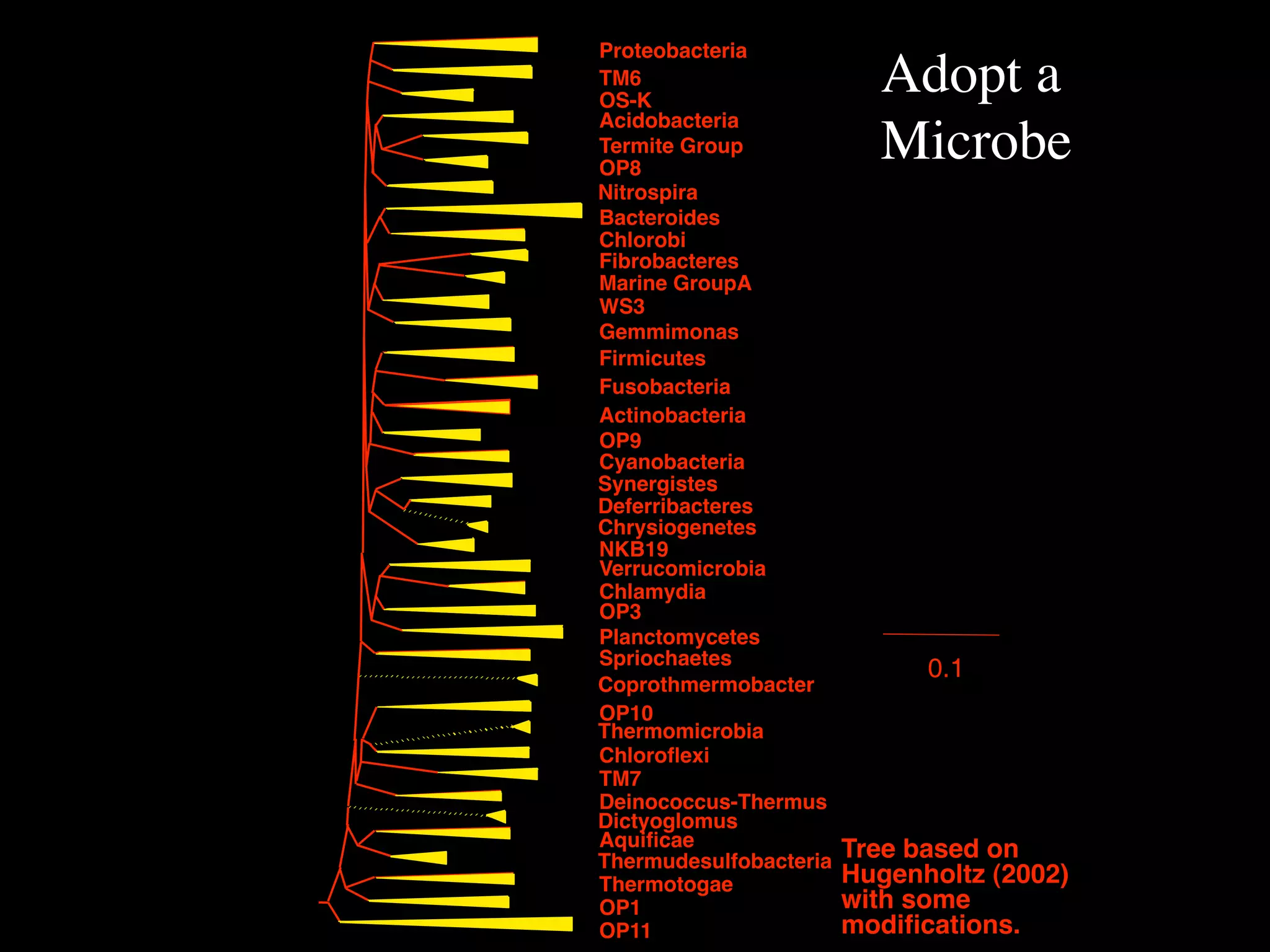
This document discusses a recent project called "A Genomic Encyclopedia of Bacteria and Archaea" (GEBA). As of 2002, genome sequences were mostly from three bacterial phyla, while some other phyla were only sparsely sampled. The GEBA project aimed to sequence a genome from each of eight underrepresented phyla to improve phylogenetic diversity. It selected one representative species from each of eight phyla for genome sequencing, including Chrysiogenes arsenatis, Coprothermobacter proteolyticus, and others. While progress, genome sampling remains biased towards certain lineages within the bacterial and archaeal tree of life.