Sulfur's Essential Role in Plants and Soils
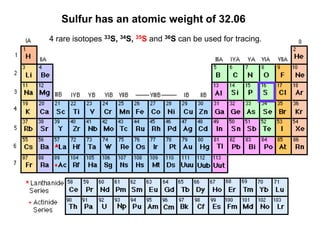
- 1. Sulfur has an atomic weight of 32.06 4 rare isotopes 33S, 34S, 35S and 36S can be used for tracing.
- 2. . . . sulfur is a devilish substance . . . Discharged from the bowels of the earth, by volcanoes or evil-smelling hot springs . . . Surely the effluent of Hell itself. – J.R. Postgate
- 3. Why is S important ? • Essential element required in relatively high concentrations • Essential component of 3 amino acids – cysteine, cystine, methionine • Disulfide bonds give structure to many proteins • Source of metabolic energy for many bacteria • Postive and negative environmental impacts – Macronutrient – Acid mine drainage, acidic deposition
- 4. The S cycle is complex
- 5. Similarities with the N cycle Many oxidation states Most of the S in soil is a component of SOM Biological transformations are important e.g., mineralization and immobilization Volatilization is a major loss pathway
- 6. Differences with the N cycle Not much S in the earth’s atmosphere naturally (most of the S in the atmosphere today is anthropogenic) Weathering of rocks is the primary source Most global S in the earth’s crust Soil concentrations range from 10s to 1000s of ppm
- 7. Sulfur Forms in Soils • Inorganic S – Sulfate dominates (SO42-) – Sulfides (S-2, flooded conditions) – Elemental S – Thiosulfates (S2O32-) – Range in oxidation states (-2 to +6) • > 90% of total S in most soils is organic
- 8. S has lots of oxidation states Sulfides, -2 Sulfide ion S2-, bisulfide ion HS-, hydrogen sulfide H2S, carbon-bonded S Polysulfide, -1 Disulfide ion S22-, pyrite (FeS2) Elemental S S0 Thiosulfate, -2 & +6 Thiosulfate ion S2O32- Sulfites, +4 Sulfite ion SO32-, sulfur dioxide SO2 Sulfate, +6 Sulfate ion SO42-, sulfuric acid H2SO4
- 9. There are many volatile biogenic S compounds Compound Formula Atmospheric Production concentration (Tg y-1) Hydrogen sulfide H2S 0.2 – 1 ppb 16.5 – 70.6 Sulfur dioxide SO2 0.2 – 5 ppb 15.0 Carbon disulfide CS2 0.1 – 0.4 ppb 3.8 – 4.7 Carbonyl sulfide COS 0.2 – 0.6 ppb 2.7 – 3.5 Methyl mercaptan CH3SH Ethyl mercaptan CH3CH2SH Dimethyl sulfide CH3SCH3 58 ppt 39.6 – 45.4 Dimethyl disulfide CH3SSCH3 1.3 – 3.4 "smell of the sea"
- 10. Is S a limiting nutrient ?
- 11. Sulfur deficiencies are increasingly common - Enforcement of clean air standards has reduced SOx emissions from power plants and industry by > 50% in the last 2 decades - The S contents of current fertilizers are far lower than those used historically. - Higher crop yields are removing higher amounts of S from soils as well as increasing the need for S.
- 13. Wet and dry S deposition is monitored throughout the US http://www2.nature.nps.gov/air/Monitoring/drymon.cfm
- 15. Sulfur emissions in Wisconsin over the last 2 decades Why did this happen?
- 16. The Cap and Trade Success Story "Cap and trade" harnesses the forces of markets to achieve cost-effective environmental protection. Markets can achieve superior environmental protection by giving businesses both flexibility and a direct financial incentive to find faster, cheaper and more innovative ways to reduce pollution. Cap and trade was designed, tested and proven here in the United States, as a program within the 1990 Clean Air Act Amendments. The success of this program led The Economist magazine to crown it "probably the greatest green success story of the past decade." (July 6, 2002).
- 18. Interpreting soil test S in Illinois Soil test S (lbs/A) RATING 0 - 12 Very low 12 - 22 Low Response > 22 unlikely
- 19. The IL Agronomy Handbook has long stated that IL crops are unlikely to be deficient in S. Experiments conducted across Illinois in the late 70s only identified a response to sulfur at 5 out of 82 site-years. Correlation between yield increases and measured S levels was low, indicating that soil test S does not reliably predict sulfur need. When soil test S levels are above 22 lbs /acre, it is very unlikely that a response to applied S will occur. When soil test S levels are below 22 lbs/acre, response to applied S is more likely (but not predictable).
- 20. It is high time for some more sulfur research in IL!
- 21. Recent S research in Central and Northeast IA The average yield response to S application (for the 6 out of 10 sites with a response) was 9 bu/acre, with a range of 5 to 13 bu/acre. The yield increases were large enough to pay for the recommended S application (15 lb S/acre for fine‐textured soils and 25 lb S/acre for coarse‐textured soils).
- 22. Sulfur Deficiency in Corn Unlike N, S is not readily Overall light green remobilized color, worse on new from older to leaves during rapid younger growth. plant parts.
- 23. Sulfur Deficiency in Wheat Overall light green color, worse on new leaves during rapid growth.
- 24. http://landresources.montana.edu/SoilFertility/Images/S/Alfalfa-S-deficiency.jpg Alfalfa is the crop most likely to respond to sulfur (S) application in Illinois. Corn has only been shown to respond in a few experiments, primarily in northwestern Illinois. Organic matter is the primary source of sulfur in soils, so soils low in organic matter are more likely to be deficient than soils high in organic matter. S deficiency is most likely on sandy soils.
- 25. Important S concepts When S is deficient, plants tend to accumulate non-protein N, which raises the N/S ratio in the plant. A N/S ratio of 9:1 to 12:1 is especially important in forages that will be used for animal feed, so that the rumen microorganisms can effectively use the N. Grasses are more able to utilize sulfate (SO42-) than legumes, grasses will tend to crowd out the legumes in S deficient pastures. Rhizobia need S to fix N. Some plants, like mustard and onion, get their smell and taste from the presence of S compounds.
- 26. Sulfur is a key factor limiting the amount of corn by-products that can be fed to cattle. Sulfur levels of most corn by-products can range from 0.4 to 0.9% S on a dry matter basis. Some liquid by-products have been tested as high as 1.5 to 2% S. Sulfur is added during both the wet and dry corn milling process, so the by-products contain additional levels above that concentrated from the original corn. Although it is based on limited research in cattle, the NRC recommends a maximum tolerable level of 0.4% of the ration dry matter for sulfur in the ration. Using that recommendation as a guide the maximum level of corn by-products would range from 30% of dry matter intake at high sulfur levels to over 70% at low levels, based strictly on the S content
- 28. So how many lbs of ammonium sulfate should be applied if your goal is 10 lbs of S? So what can you apply if your soil needs S? Ammonium sulfate (21-0-0) ? Ammonium thiosulfate (12-0-0) – 26% S Potassium sulfate (0-0-50) - 18% S Sul-Po-Mag (0-0-22-S) - 23% S, 11% Mg Gypsum aka calcium sulfate - 17% S Elemental S – 100% S (ES95, ES90, ES85) Animal manures – 0.1-0.3% of DM What is the sulfur content of ammonium sulfate? Chemical formula = (NH4)2SO4 Molecular weight = 132.1 g/mol Atomic weight of S = 32.1 g/mol 32.1/132.1*100 = 24% S
- 29. Example of an elemental S product ?
- 30. Some elemental S products degrade more rapidly
- 31. When mixed with other fluid fertilizers and applied as a concentrated band, ATS can enhance micronutrient availability, inhibit urease activity, inhibit nitrification and improve availability of P ATS is a weak inhibitor compared to N-Serve and Agrotain