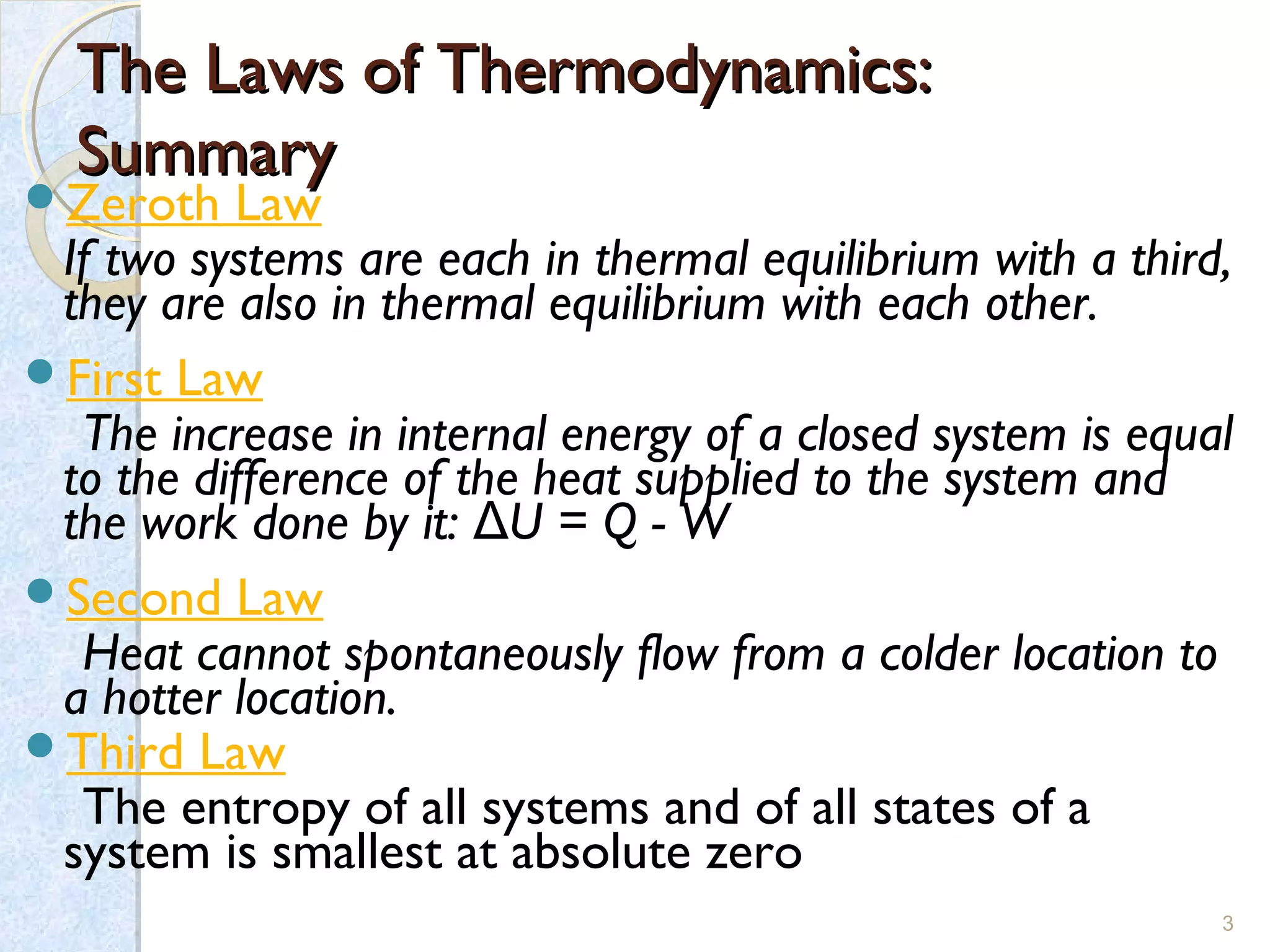
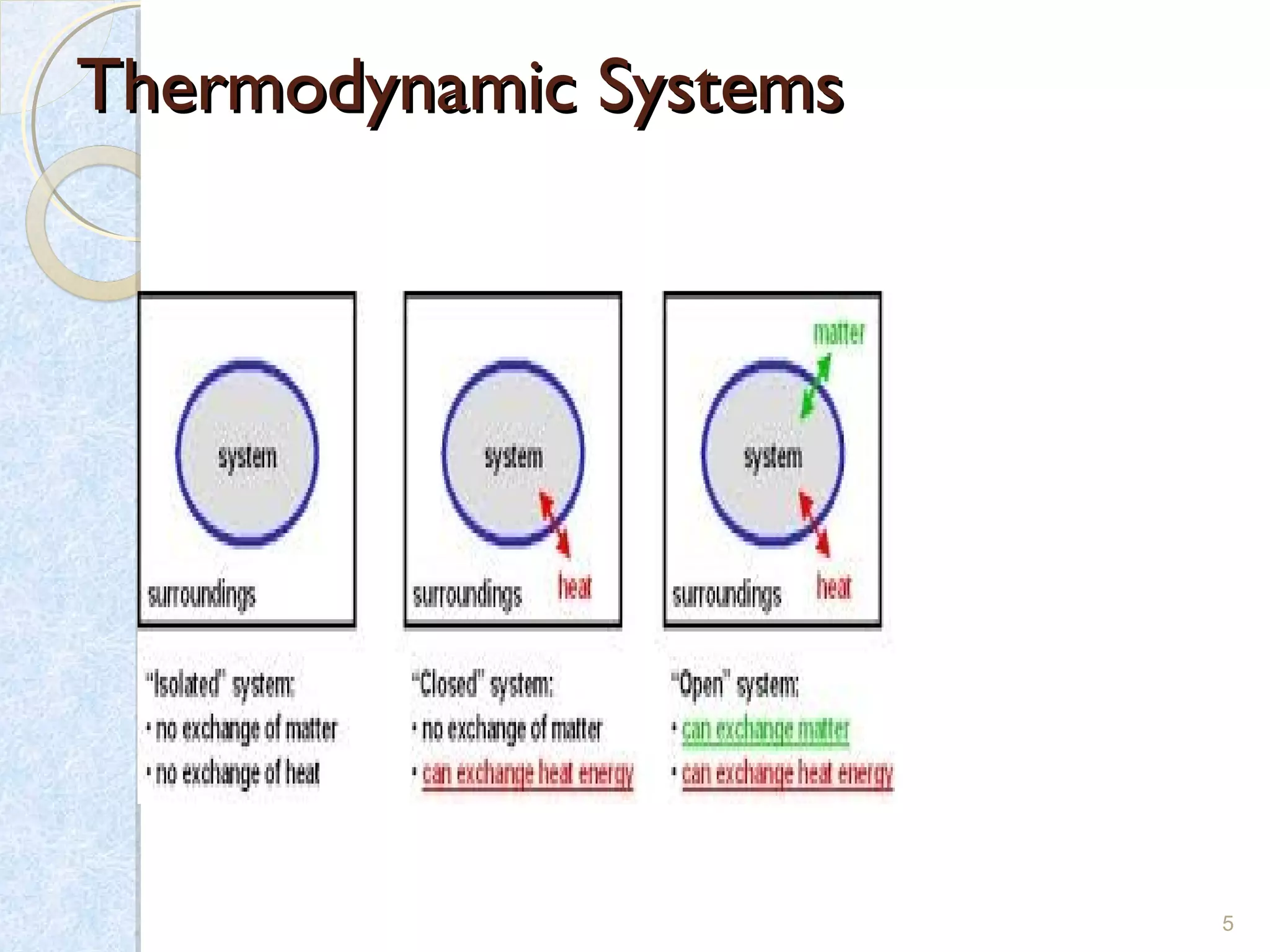
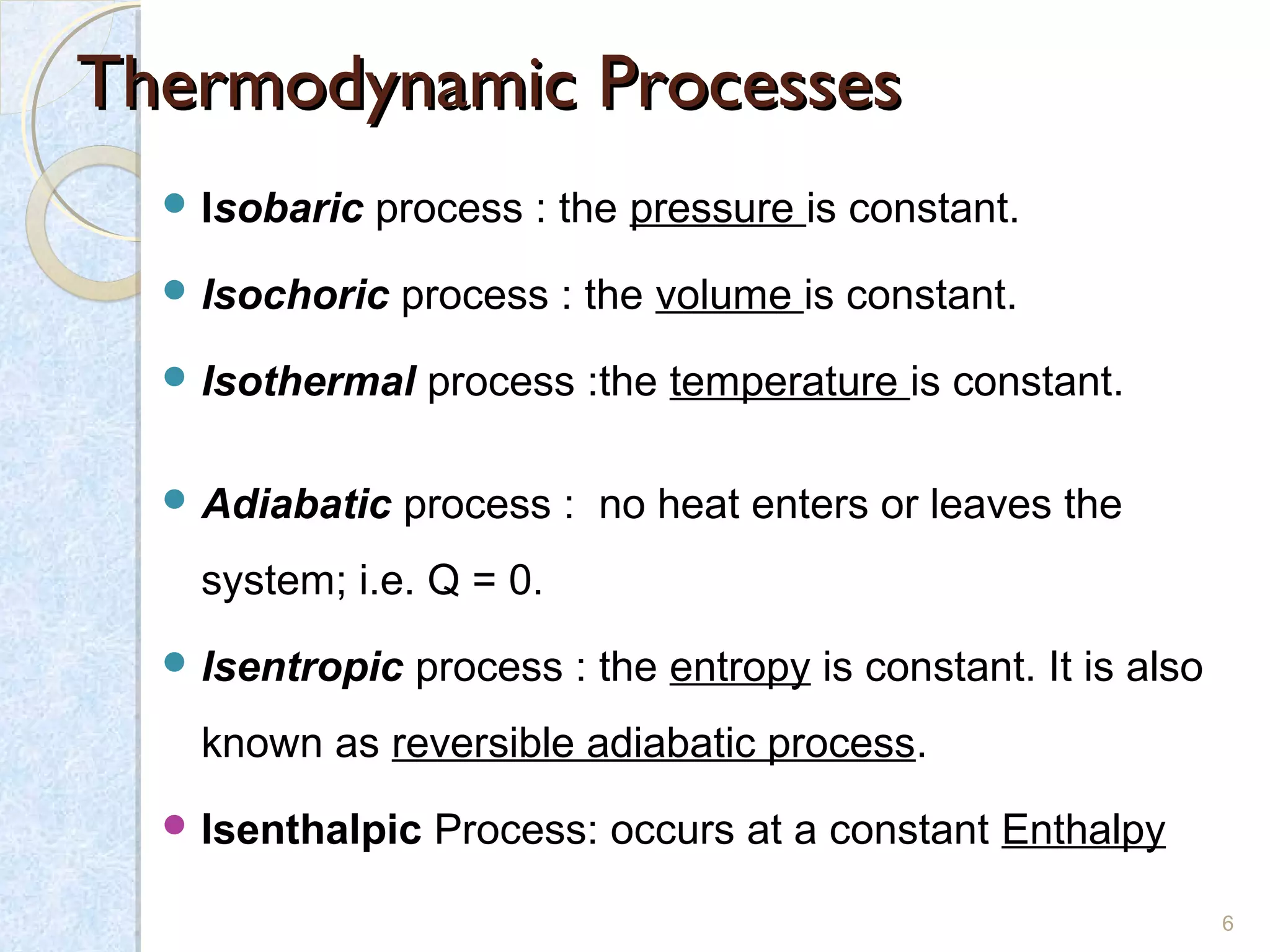
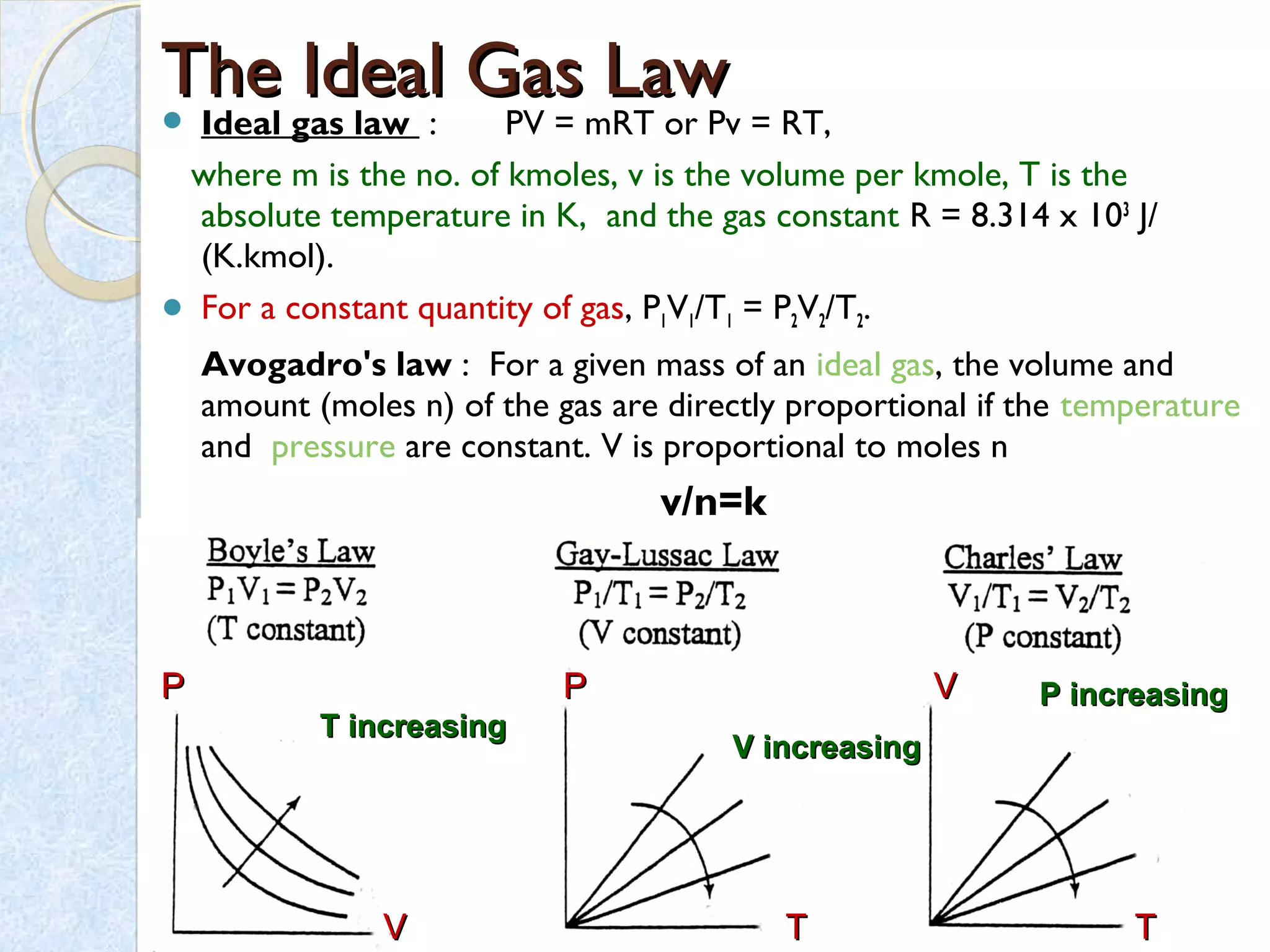
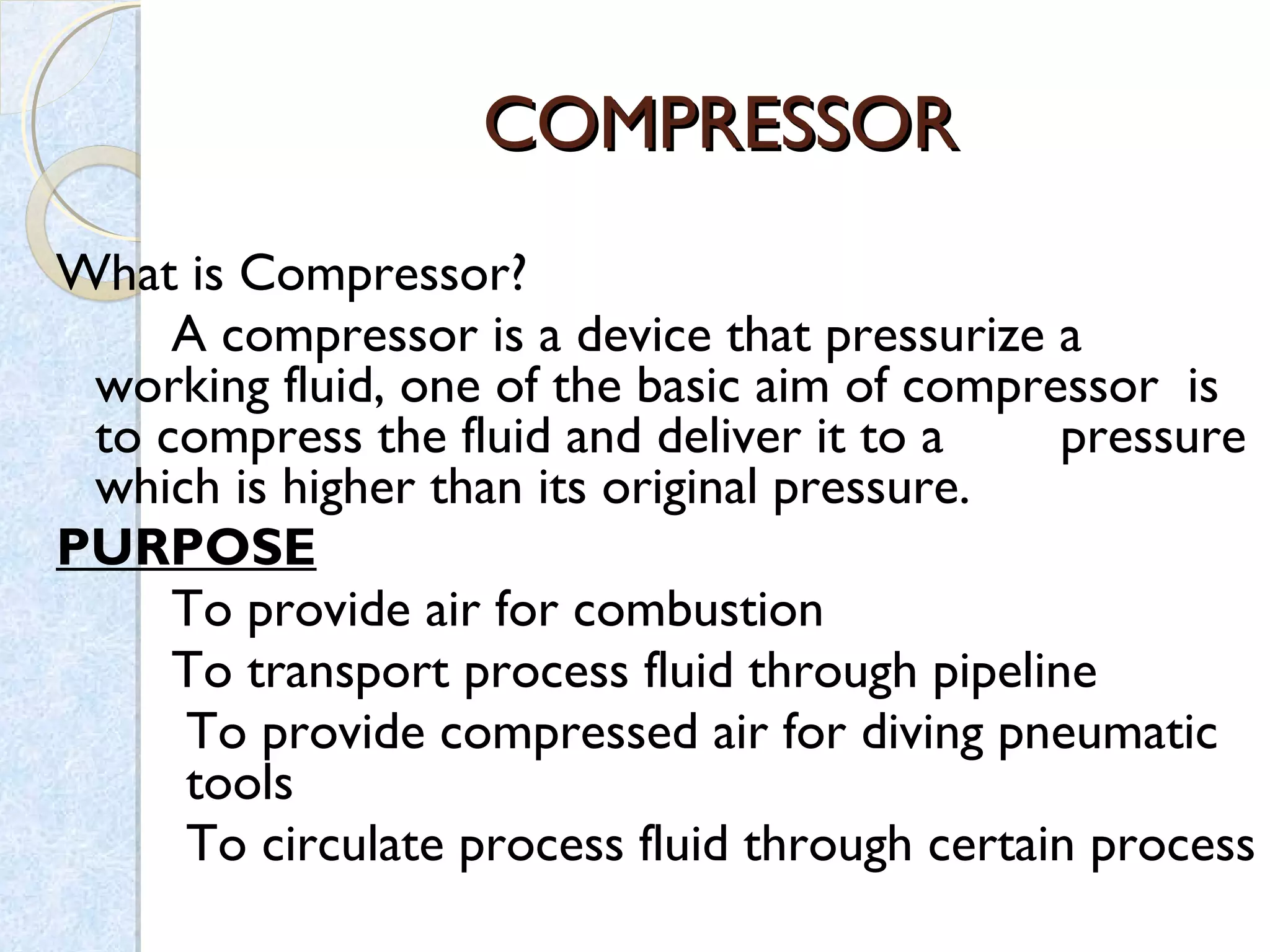
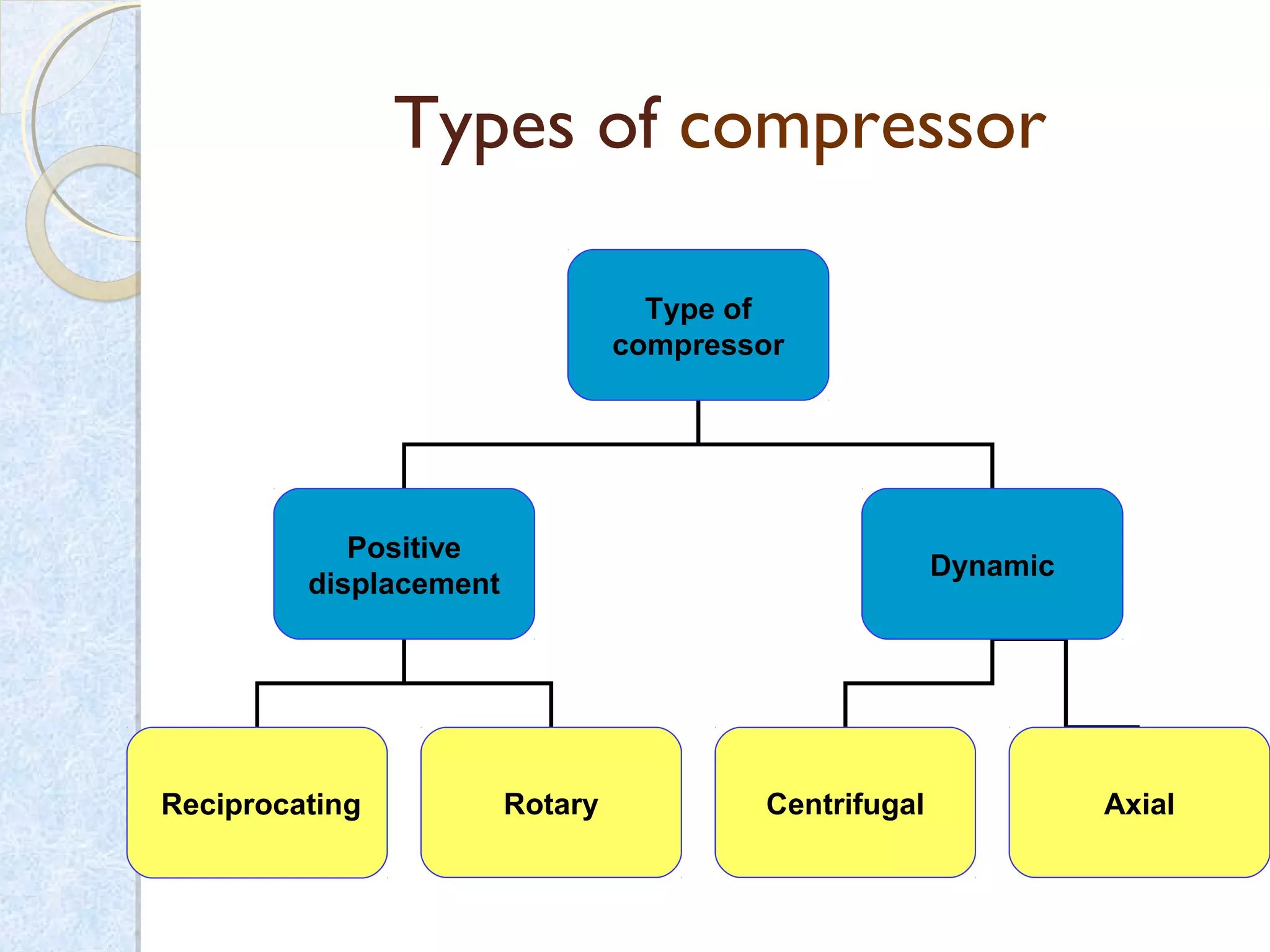
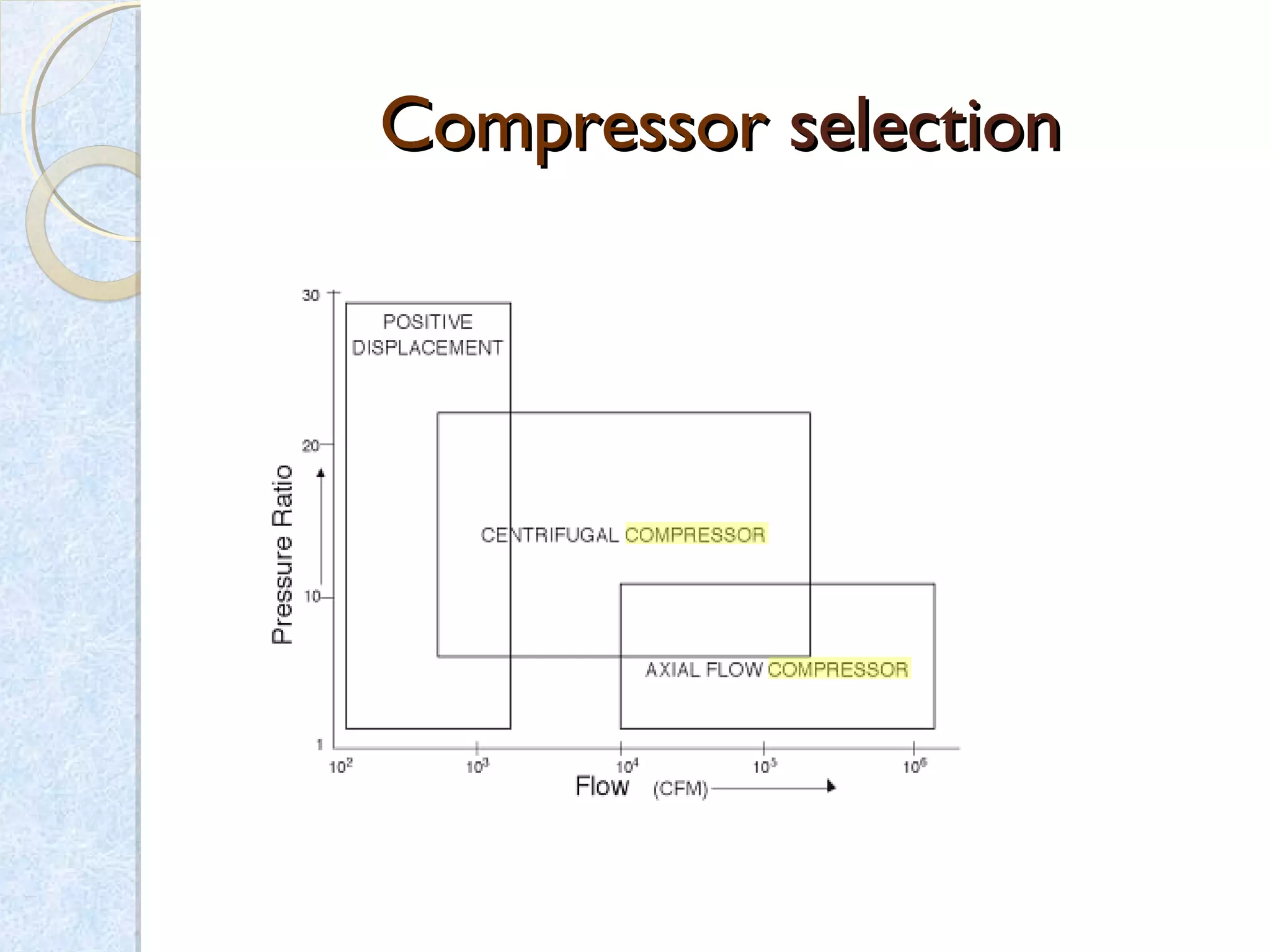
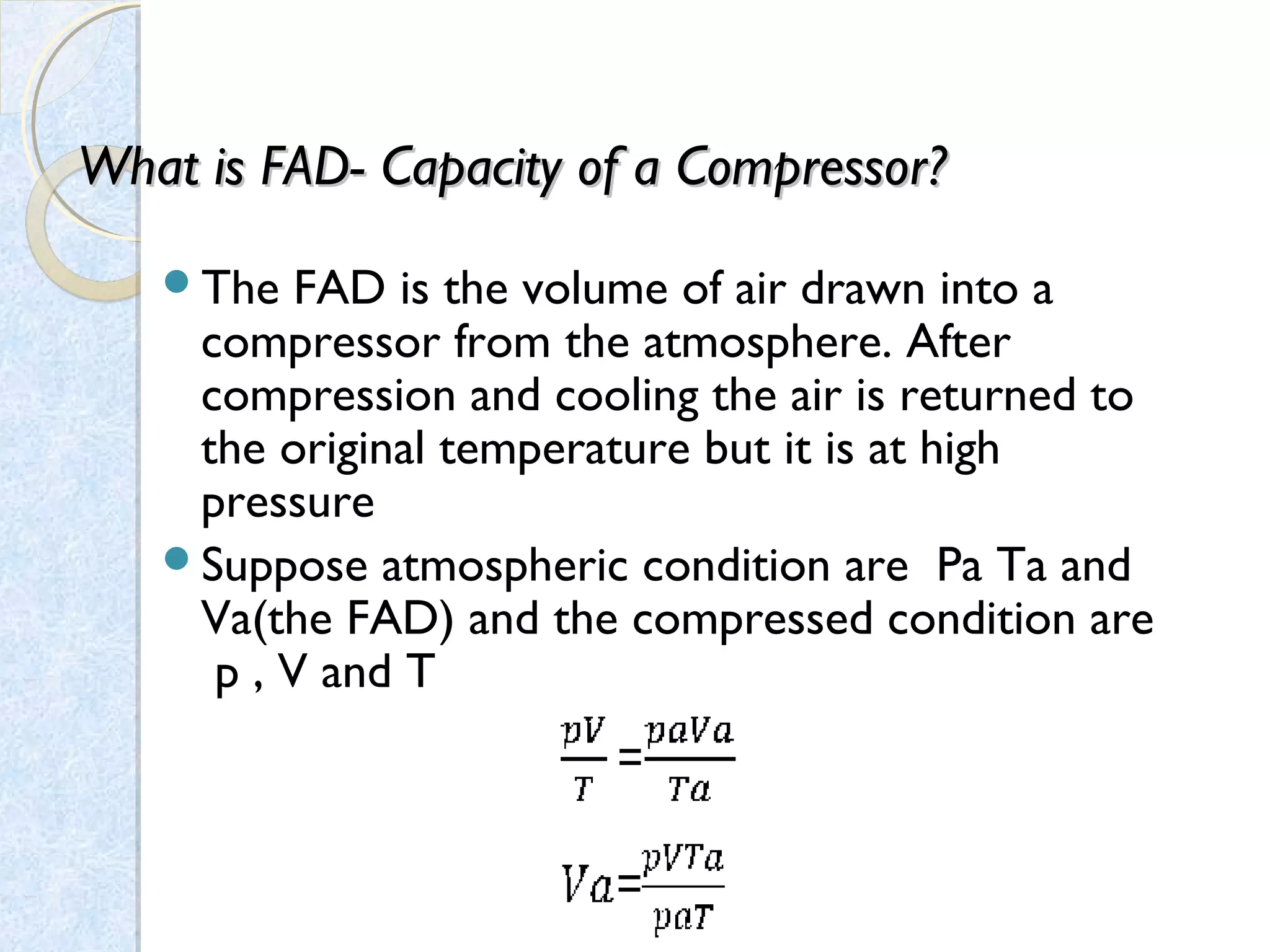
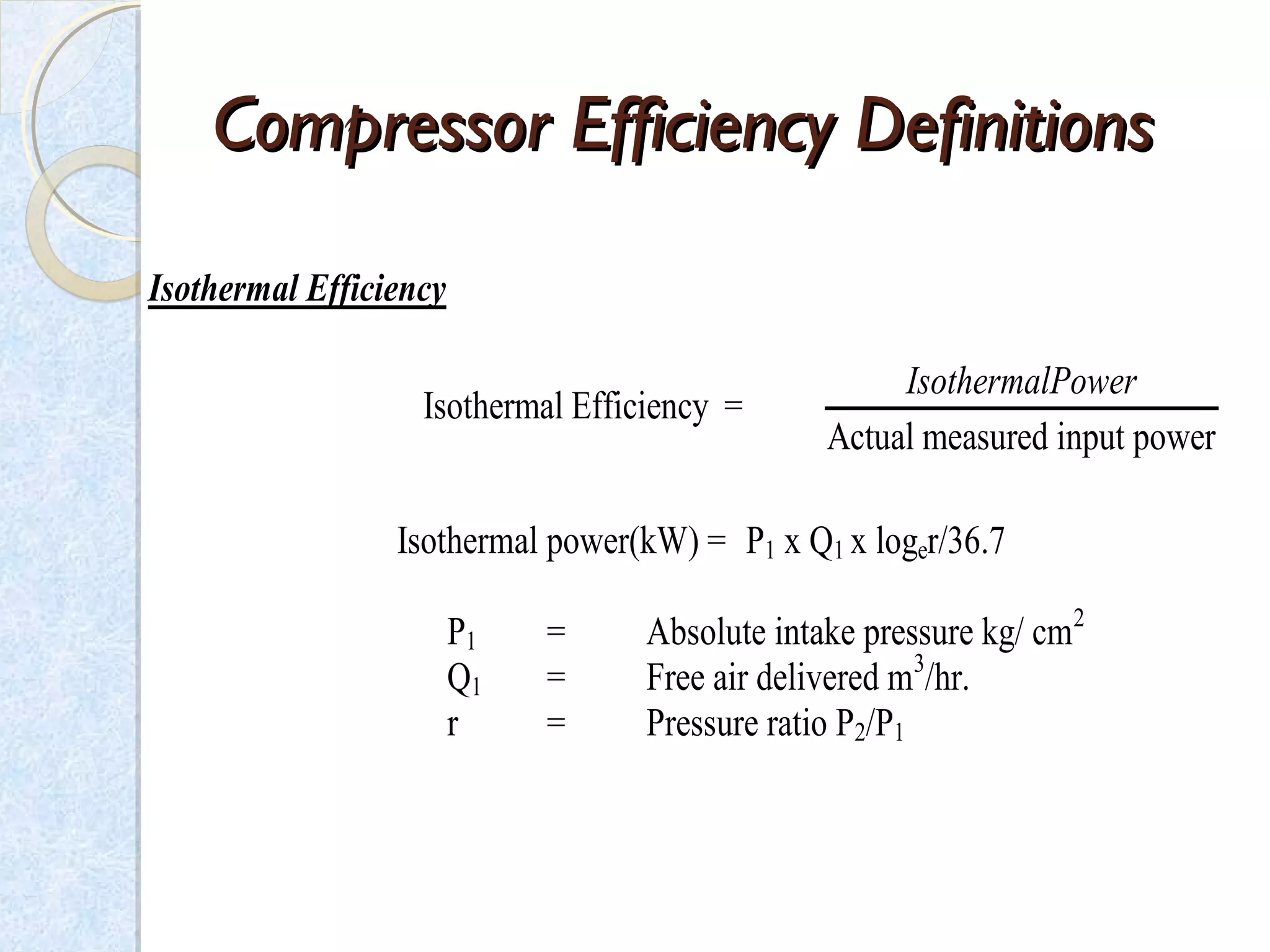
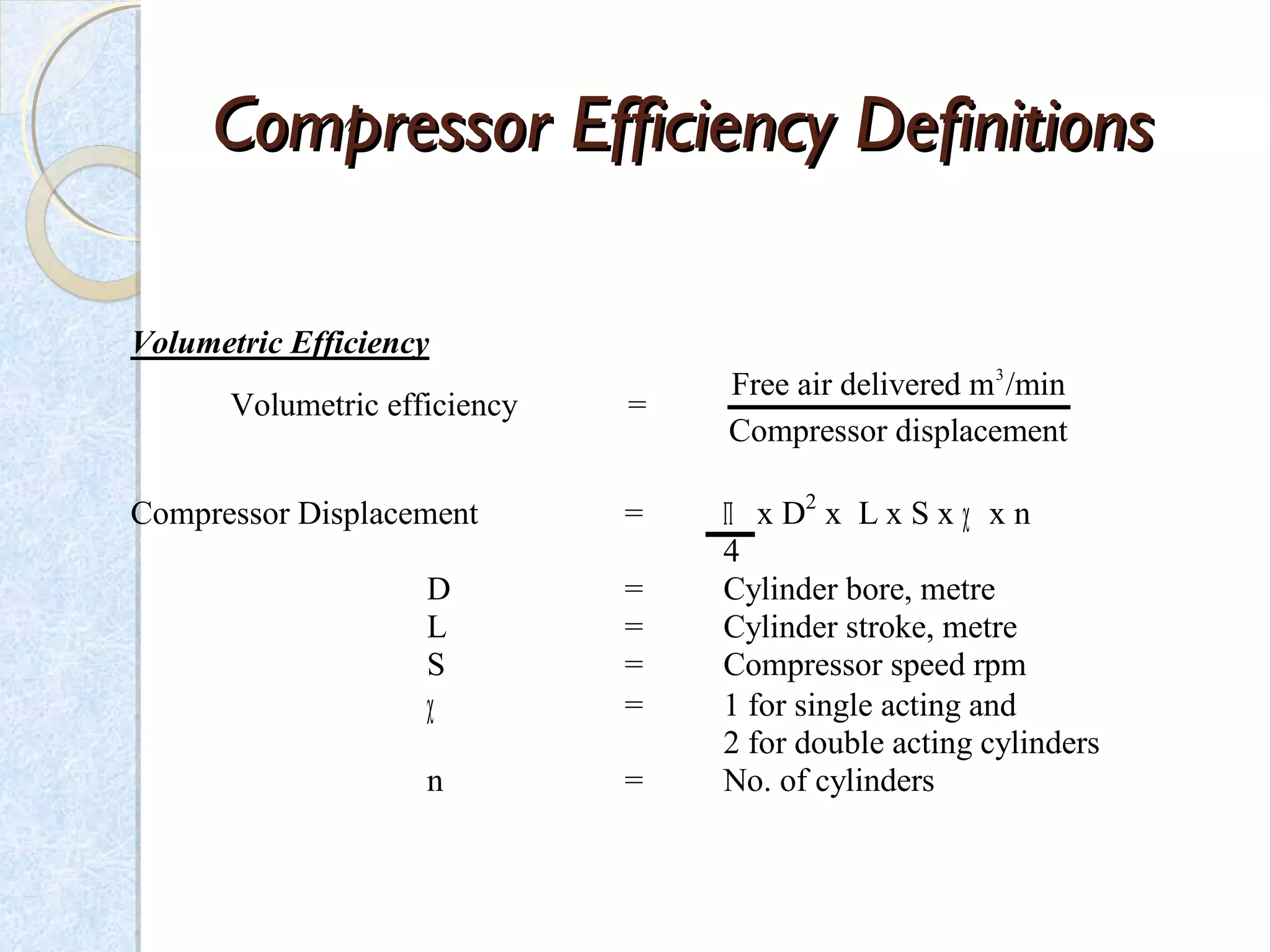
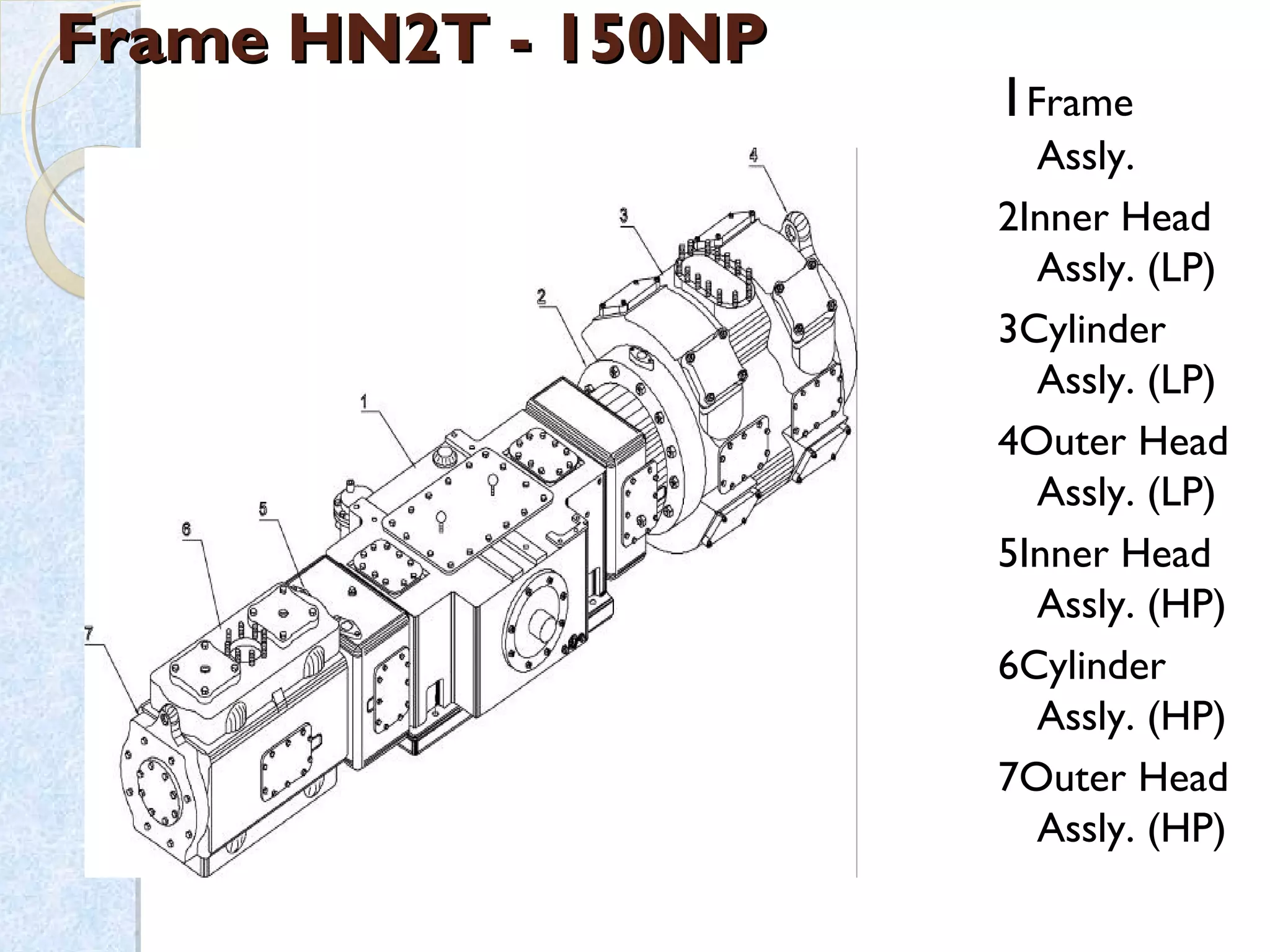
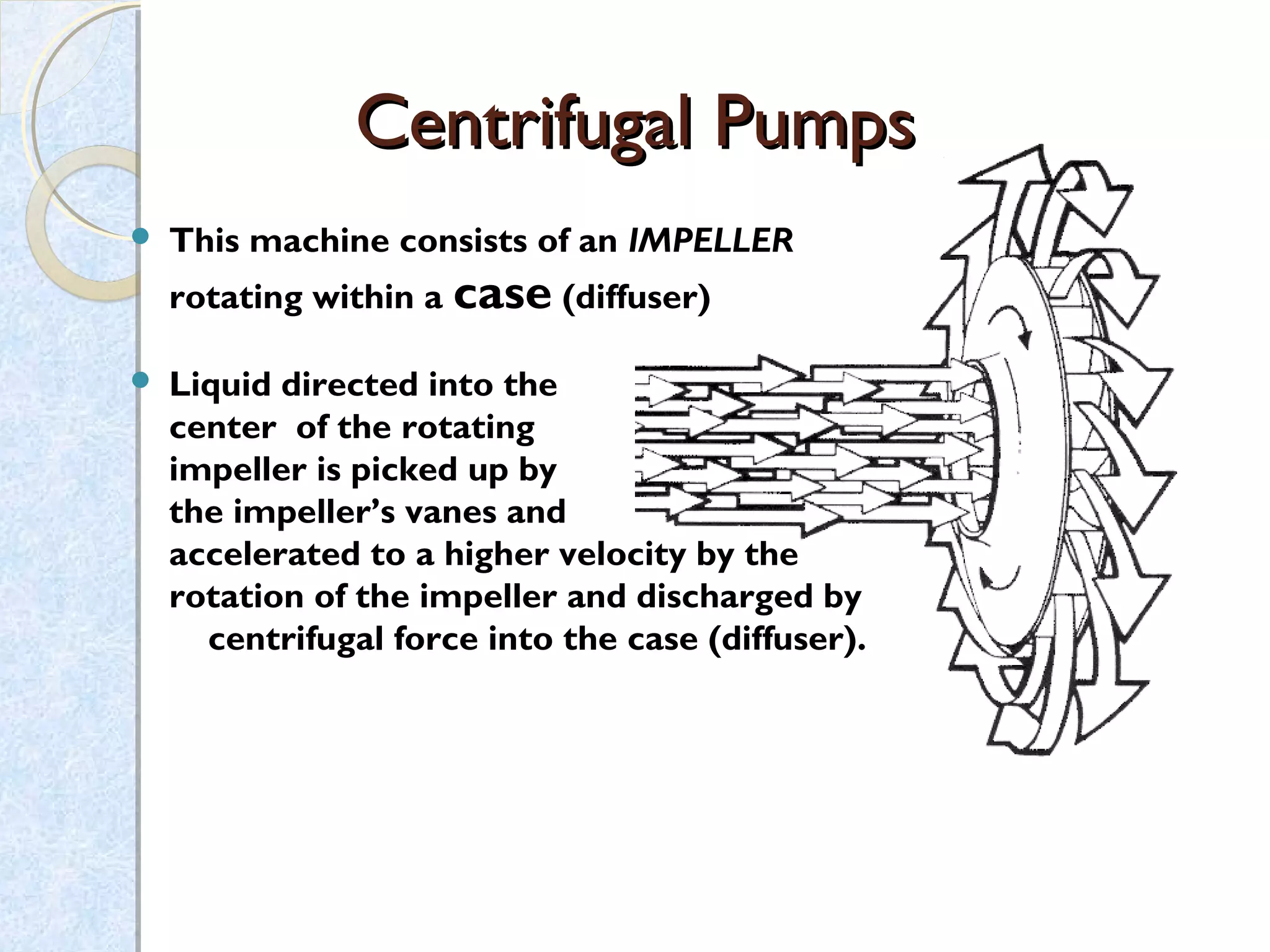
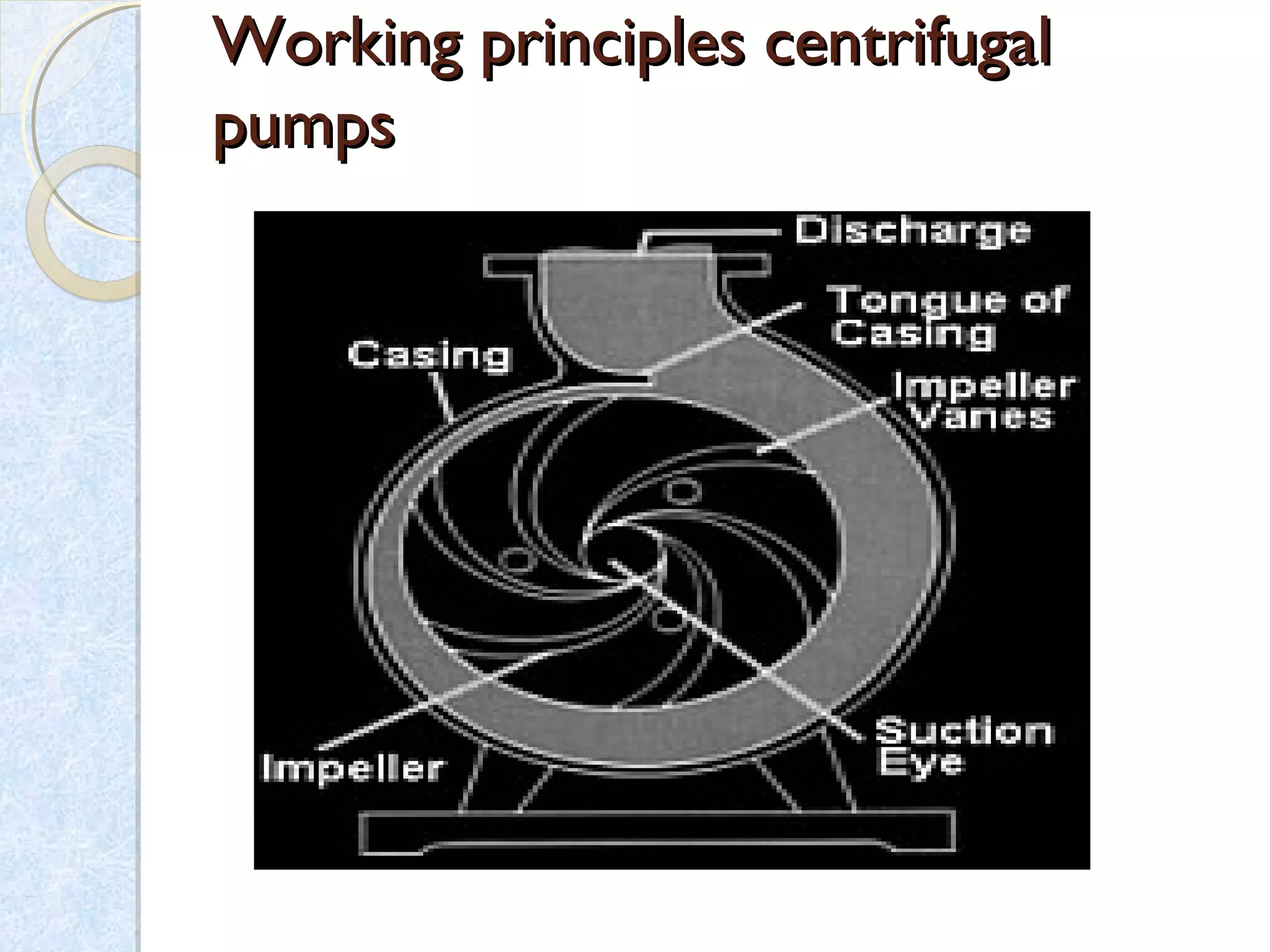
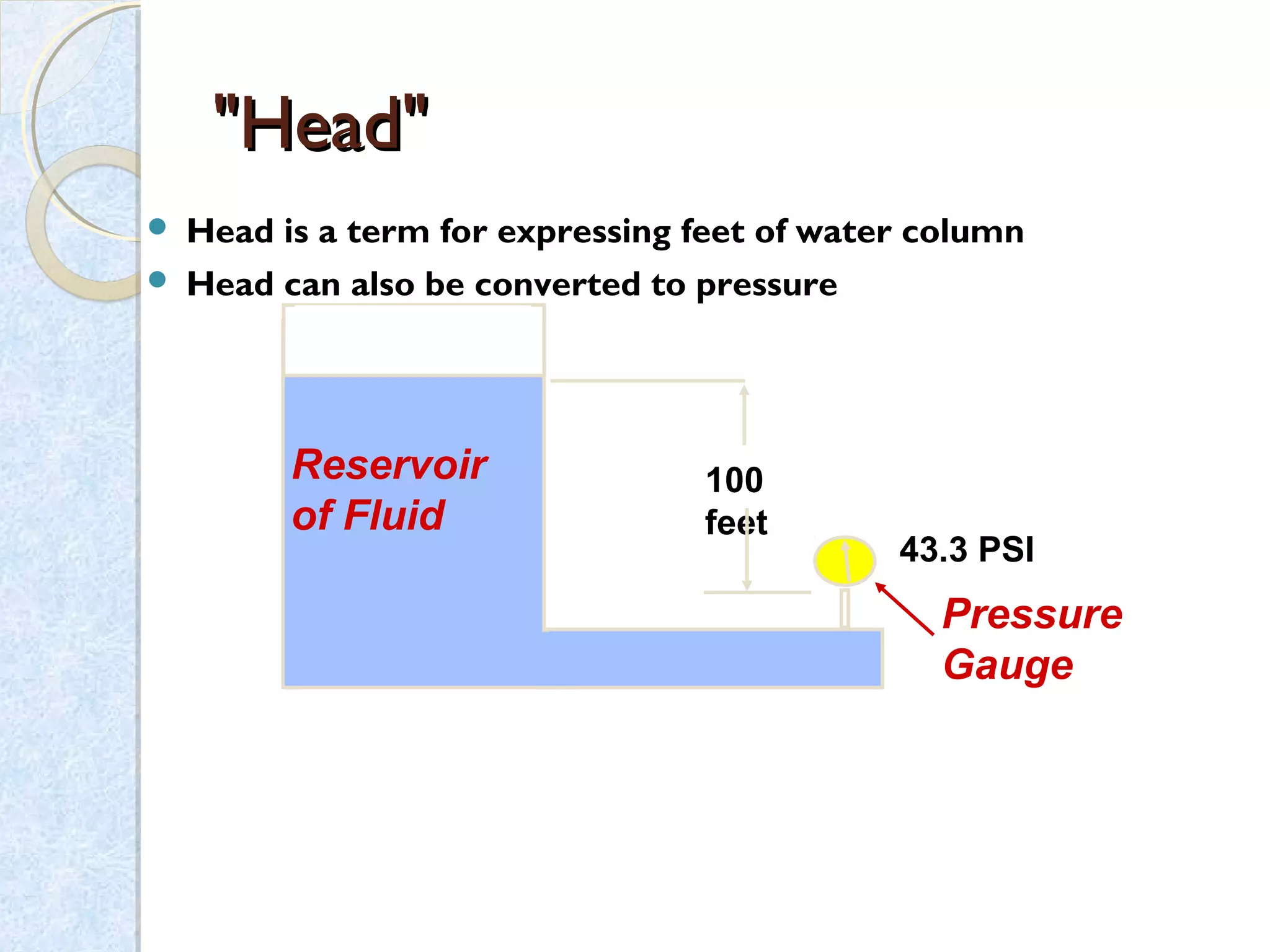
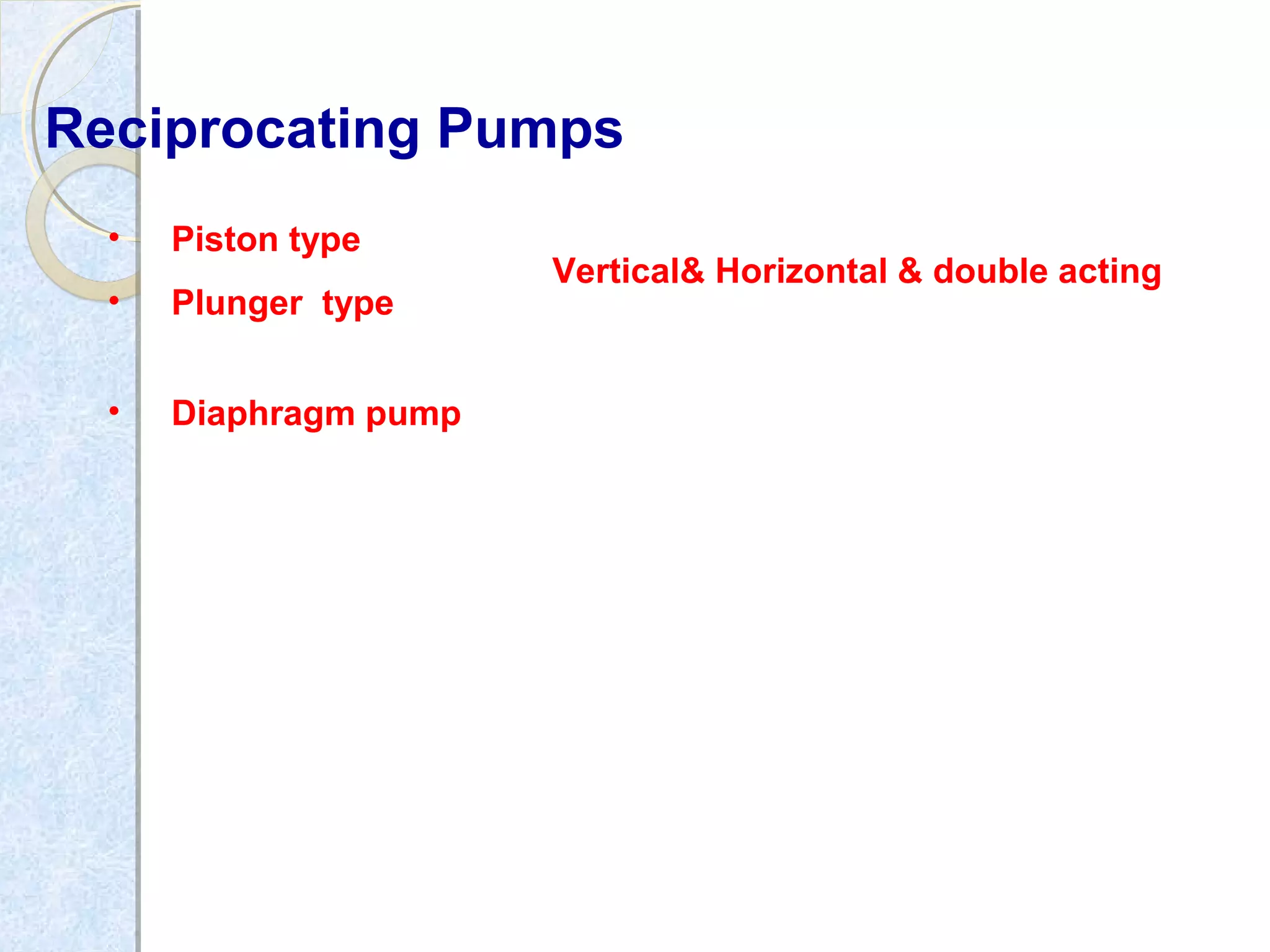
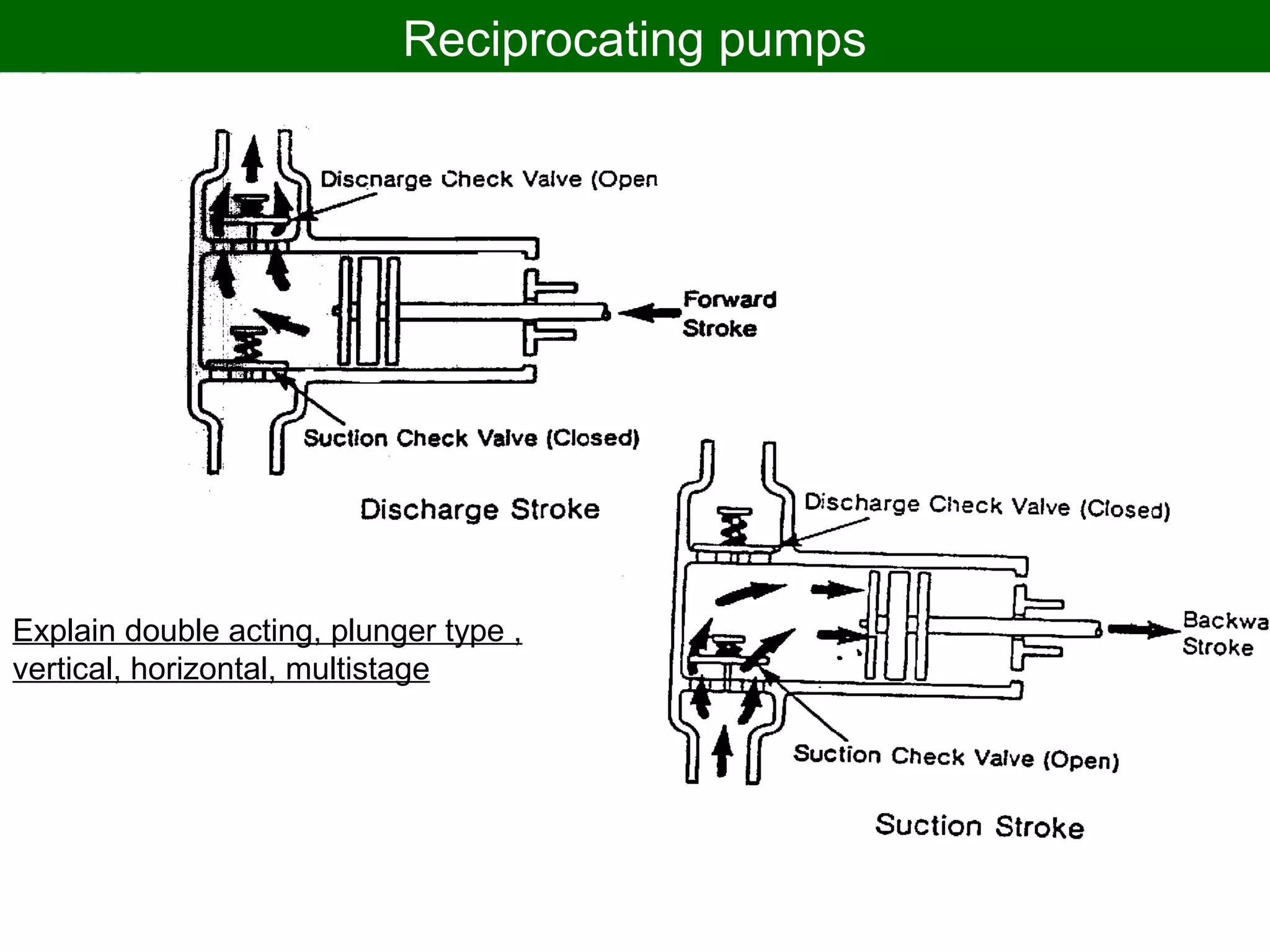
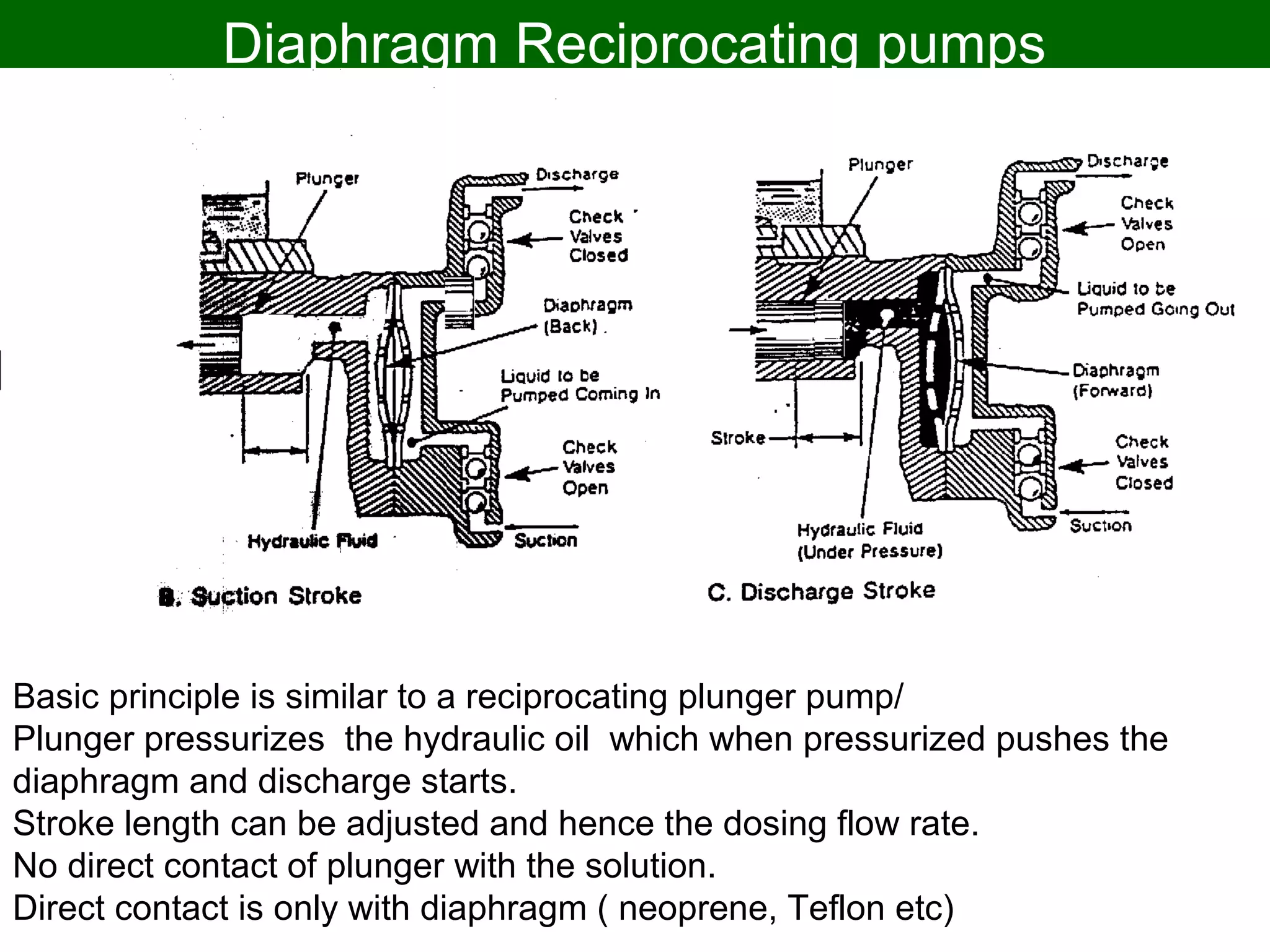
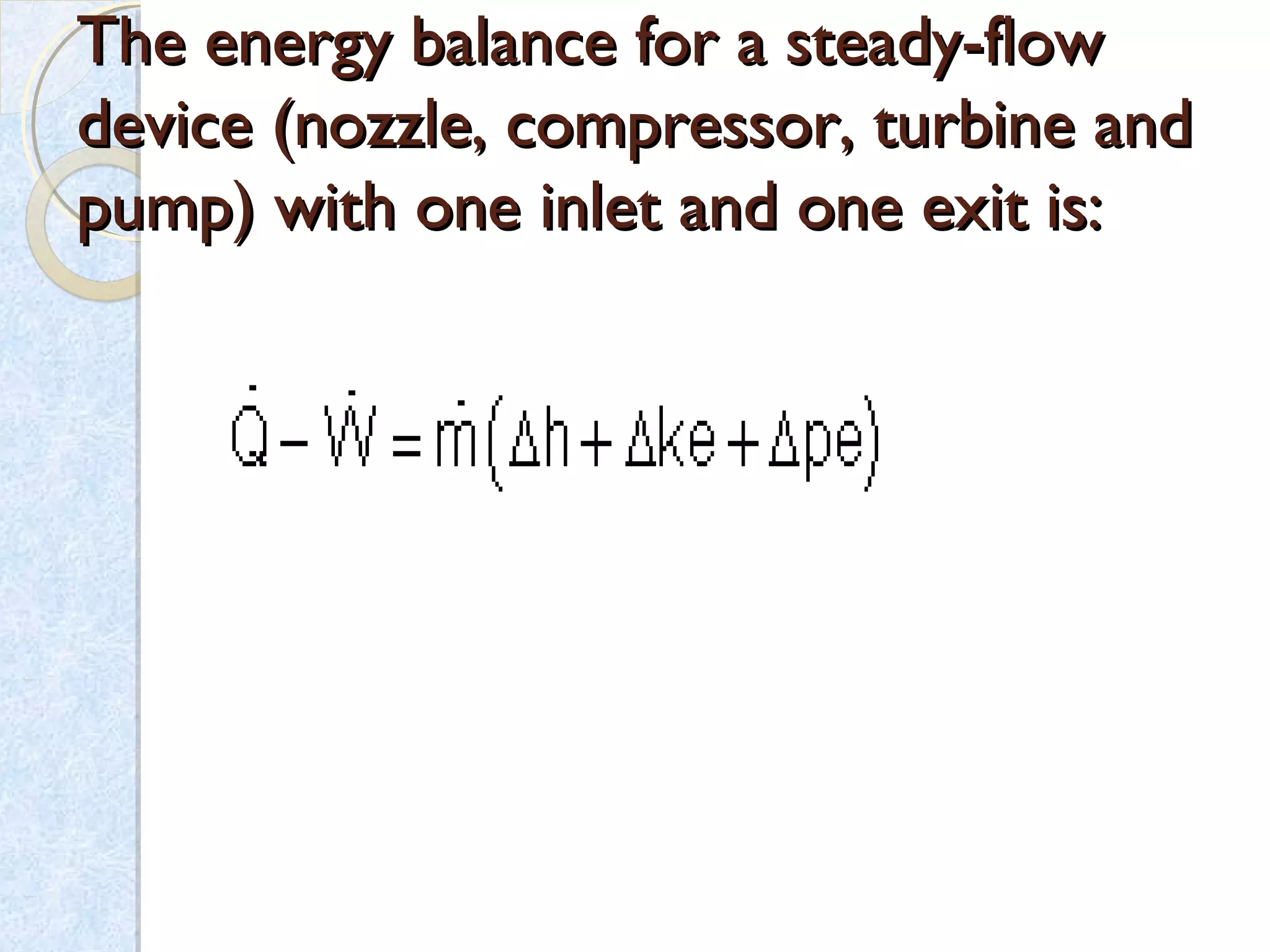Thermodynamics deals with the effects of work, heat and energy on systems. It considers macroscopic and microscopic changes. The laws of thermodynamics are:
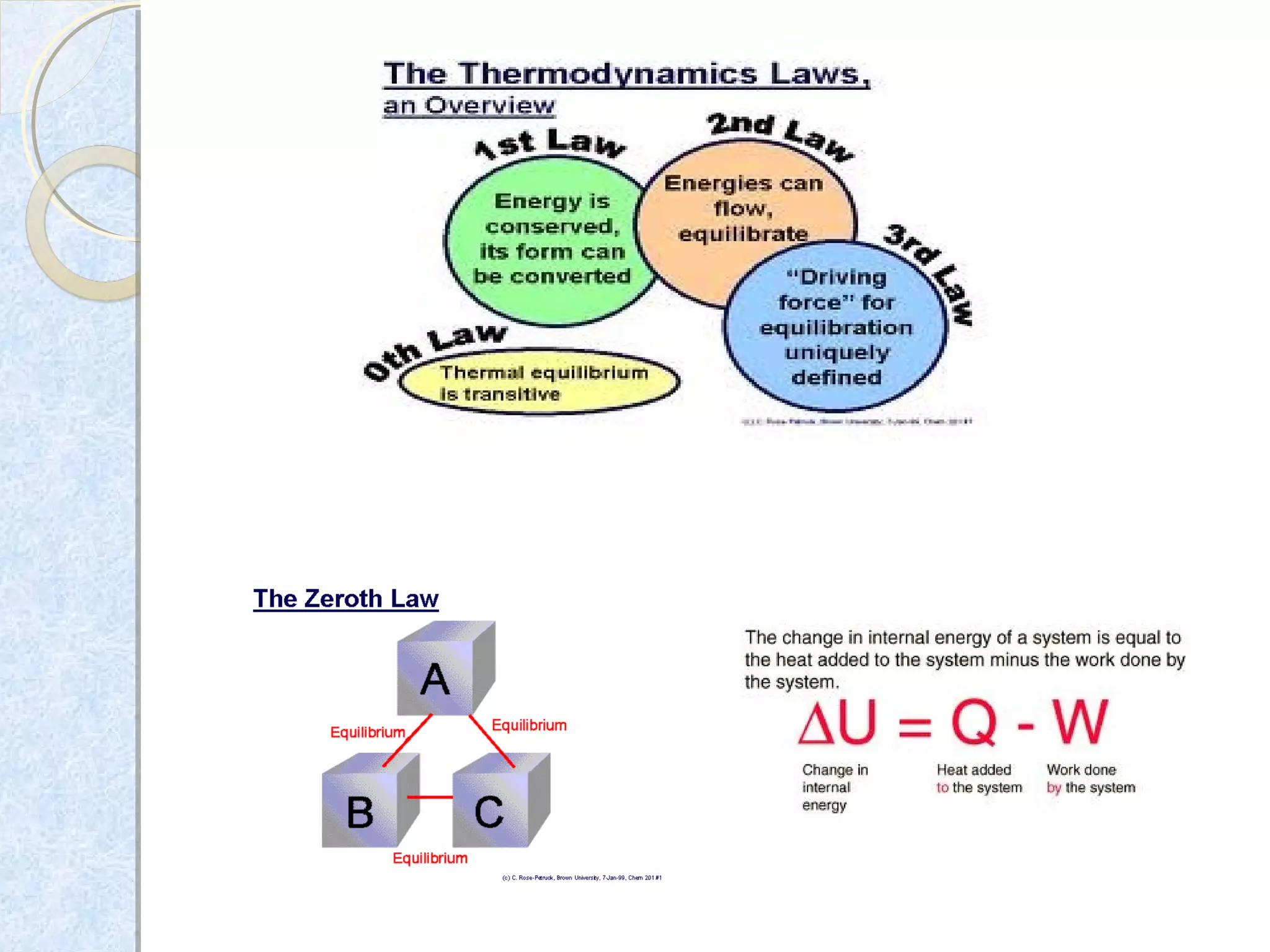
1) Zeroth law - If two systems are in thermal equilibrium with a third, they are in equilibrium with each other.
2) First law - The change in internal energy of a closed system equals the heat supplied minus the work done.
3) Second law - Heat cannot spontaneously flow from a cold body to a hot body.
4) Third law - The entropy of a system approaches a constant value as the temperature approaches absolute zero.