Diffusion and Osmosis
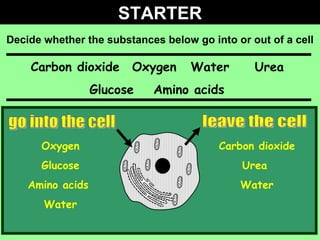
- 1. STARTER Decide whether the substances below go into or out of a cell go into the cell leave the cell Carbon dioxide Oxygen Water Urea Glucose Amino acids Oxygen Glucose Amino acids Water Carbon dioxide Urea Water
- 4. Moving into and out of cells Partially permeable membrane Allows some substances to cross, but not others. It’s selective! Two process which substances can move into or out of cells are DIFFUSION & OSMOSIS Complete page 9
- 5. DIFFUSION WATCH THE TEACHER DEMONSTRATION
- 6. Particle picture of diffusion All particles that are moving have KINETIC ENERGY When they collide into another particle they transfer this kinetic energy causing them to move too. In this example, this movement of particles mixes the water and dye together
- 7. DIFFUSION IN ACTION cotton wool red litmus paper glass tube Ammonia solution in pipette
- 8. DIFFUSION IN ACTION drop of ammonia solution (alkali) placed on cotton wool cotton wool red litmus paper glass tube
- 9. DIFFUSION IN ACTION cotton wool red litmus paper glass tube
- 10. DIFFUSION IN ACTION cotton wool red litmus paper glass tube
- 11. DIFFUSION IN ACTION cotton wool red litmus paper glass tube
- 12. DIFFUSION IN ACTION cotton wool red litmus paper glass tube
- 13. DIFFUSION IN ACTION cotton wool red litmus paper glass tube
- 14. DIFFUSION IN ACTION cotton wool red litmus paper glass tube
- 15. DIFFUSION IN ACTION cotton wool red litmus paper glass tube
- 16. DIFFUSION IN ACTION cotton wool red litmus paper glass tube
- 17. DIFFUSION IN ACTION cotton wool red litmus paper glass tube
- 18. DIFFUSION IN ACTION cotton wool red litmus paper glass tube
- 19. DIFFUSION IN ACTION cotton wool red litmus paper glass tube
- 20. DIFFUSION IN ACTION cotton wool red litmus paper glass tube
- 21. High concentration Low concentration DIFFUSION THE BIGGER THE CONCENTRATION DIFFERENCE THE FASTER THE DIFFUSION This side is constantly added to - maintains high concentration This side is constantly removed - maintains low concentration Concentration Gradient Complete pages 9, 10 & 11
- 23. Particle animation of osmosis
- 24. High Concentration WATER Low Concentration WATER OSMOSIS THE BIGGER THE CONCENTRATION DIFFERENCE THE FASTER THE OSMOSIS LOTS OF DISSOLVED PARTICLES LITTLE DISSOLVED PARTICLES Why osmosis happens Complete pages 11, 12, 13, 14 & 15
- 25. Osmosis in animal cells Hypertonic solution Hypotonic solution BURSTS shrinks
- 26. Osmosis in plant cells Hypertonic solution Hypotonic solution
- 27. Investigation 0.1 0.2 0.3 0.4 0.5 0.5 0.4 0.3 0.2 0.1 End mass (g) Initial mass (g) Sol. (M)