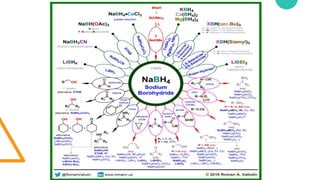
1. The sodium borohydride production process involves 7 steps, including steam reforming methane to make hydrogen, electrolysis of sodium chloride to make sodium metal, and combining sodium hydride and trimethylborate to make sodium borohydride.
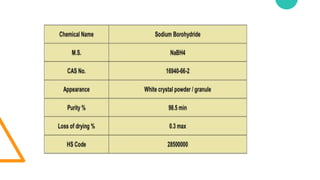
2. Sodium borohydride has a variety of end uses including in pharmaceuticals, pulp and paper, metal recovery, textiles, and organic chemical purification.
3. The global sodium borohydride market was valued at $1.6 billion in 2019 and is projected to reach $2.4 billion by 2027, growing at a CAGR of 5.1% due to its use as a reducing agent in industry and laboratories.