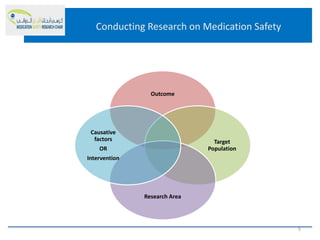
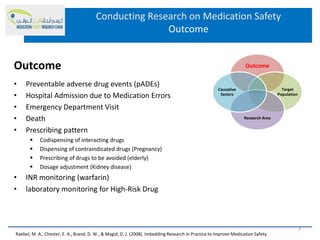
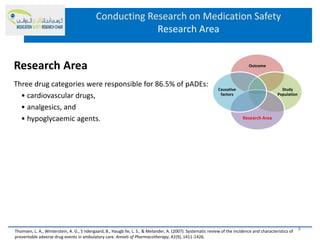
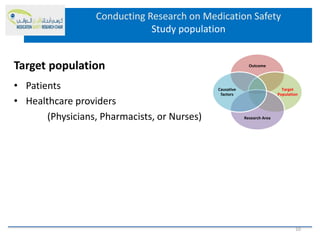
This document discusses opportunities for medication safety research. It defines medication errors and outlines the National Coordinating Council for Medication Error Reporting and Prevention's classifications of medication errors. Research on medication safety is important to understand error causes and reduce errors. Key areas for research include outcomes, target populations, factors that cause errors, and intervention studies. Quantitative research using large databases can study prescribing patterns and adverse drug events while qualitative research explores human, process, and system factors contributing to errors. The document provides examples of potential medication safety studies.