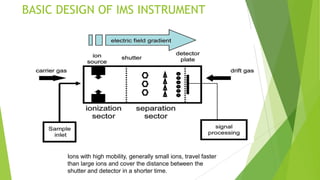
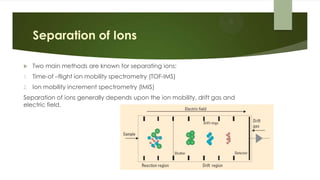
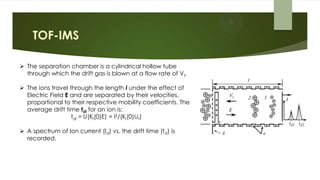
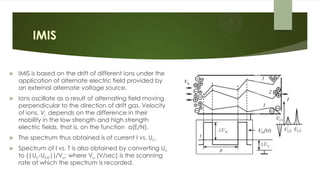
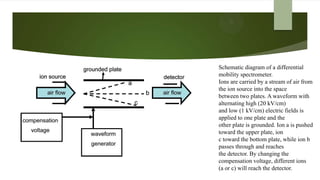
Ion mobility spectrometry (IMS) is an analytical technique used to detect explosives and other chemicals. It works by ionizing vapor samples, separating the ions based on their mobility in an electric field, and detecting the ions. IMS provides fast, sensitive detection and is used in security applications like airports and public events. The document discusses the principles and components of IMS, including ionization methods, ion separation techniques, and factors that determine the resolution, sensitivity and detection limits of IMS systems. Commercial applications of IMS are highlighted, such as explosive detectors, air quality monitors, and portals used to screen people for explosive residues.







![K = [3∙e∙(2∙π)½ (1+α)]/[16∙N∙(μ.k.Teff)½ ∙ΩD∙ (Teff)]
Here, e: charge of an electron,
α : correction factor (usually below 0.02 under low electric field conditions),
N : number density of drift gas molecules.
μ : reduced mass of the ion mass(m) and drift gas (M) molecules [μ=m*M/(m+M)],
k : the Boltzmann constant,
Teff : effective temperature of the ion
ΩD : effective cross section for collision of the ion with the drift gas molecules.
UNIT OF MOBILITY COEFFICENT = cm2 V-1 sec-1](https://image.slidesharecdn.com/ionmobilityspectrometer-140506213617-phpapp02/85/Ion-mobility-spectrometery-9-320.jpg)