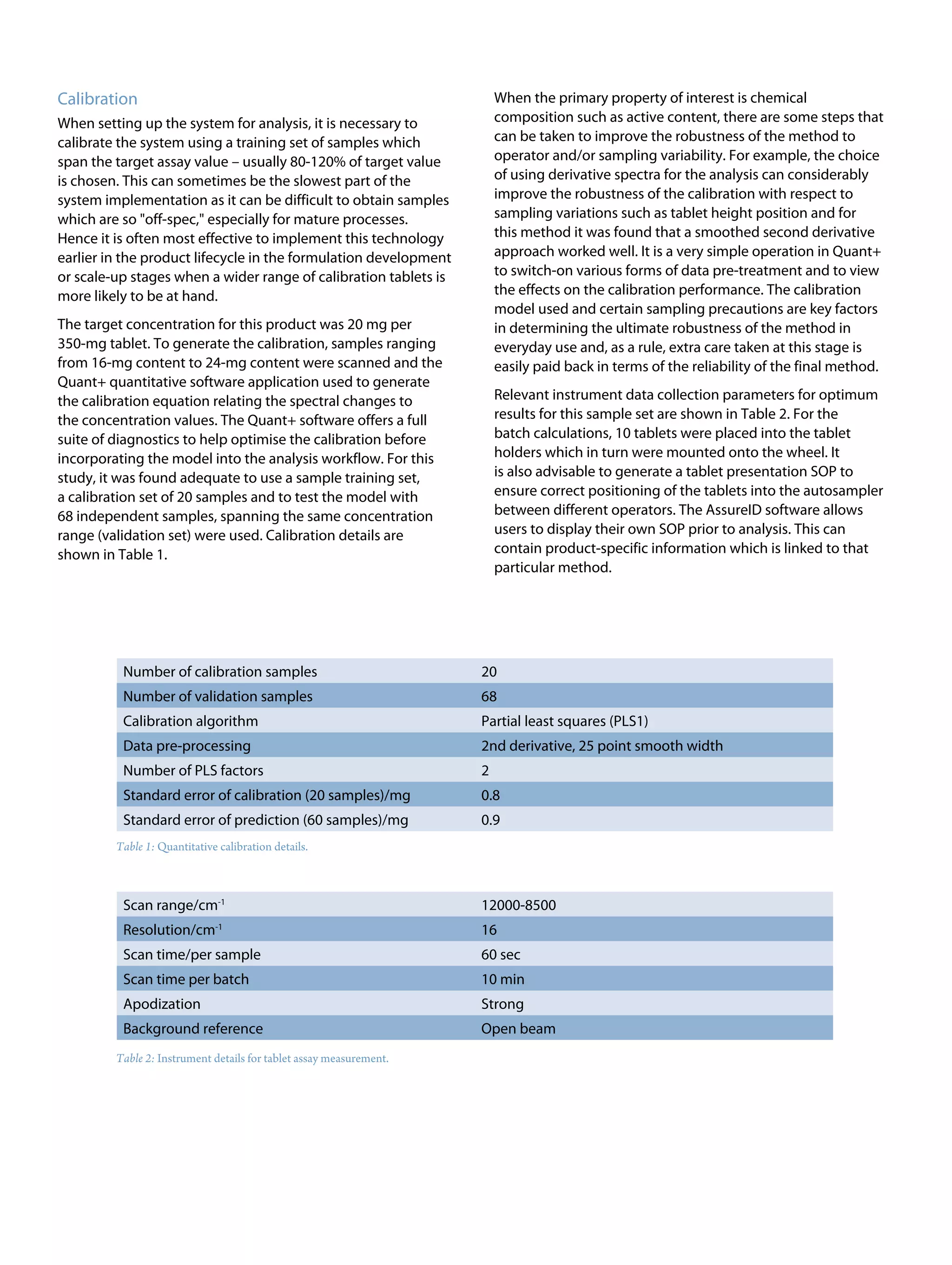
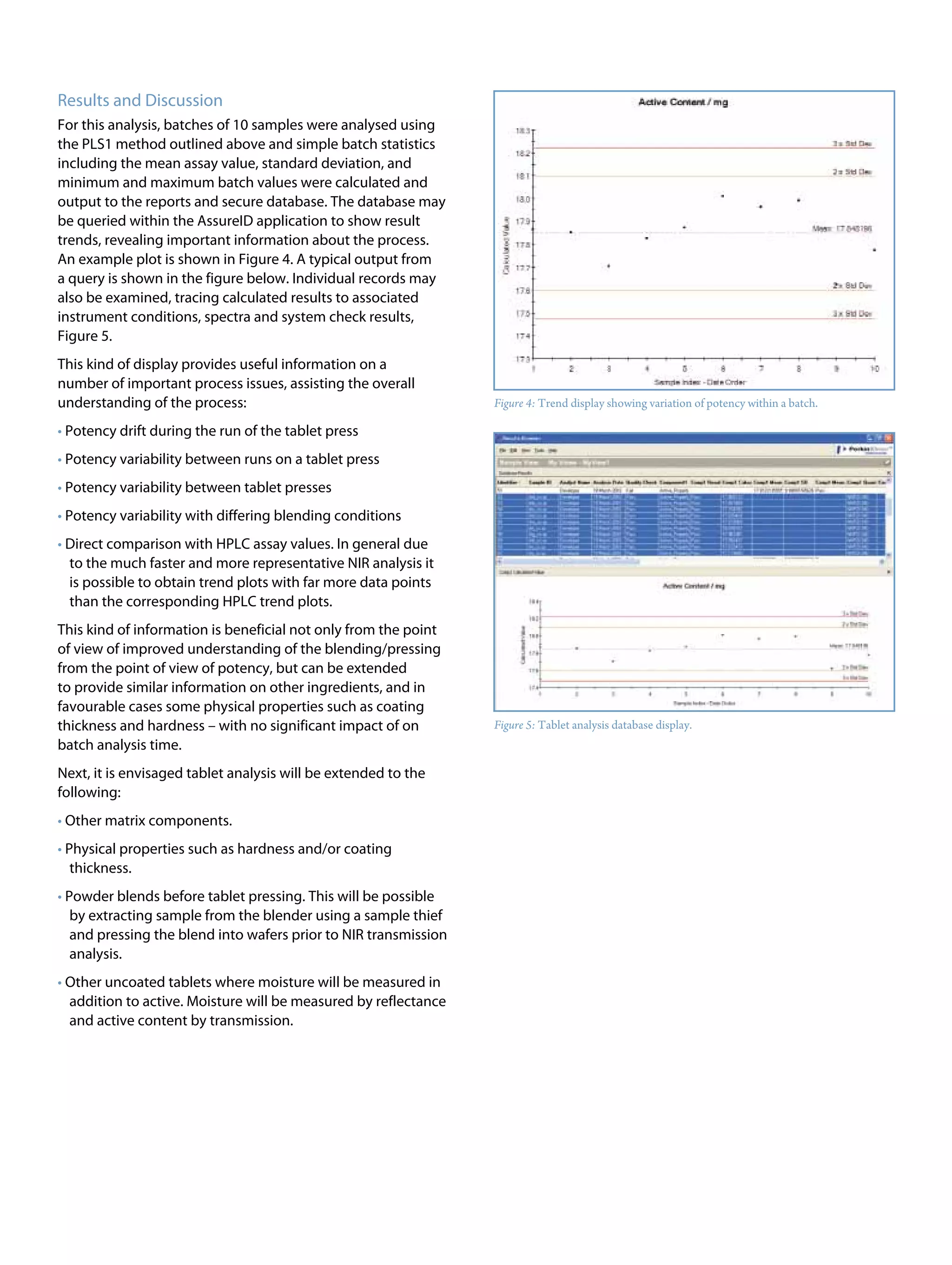
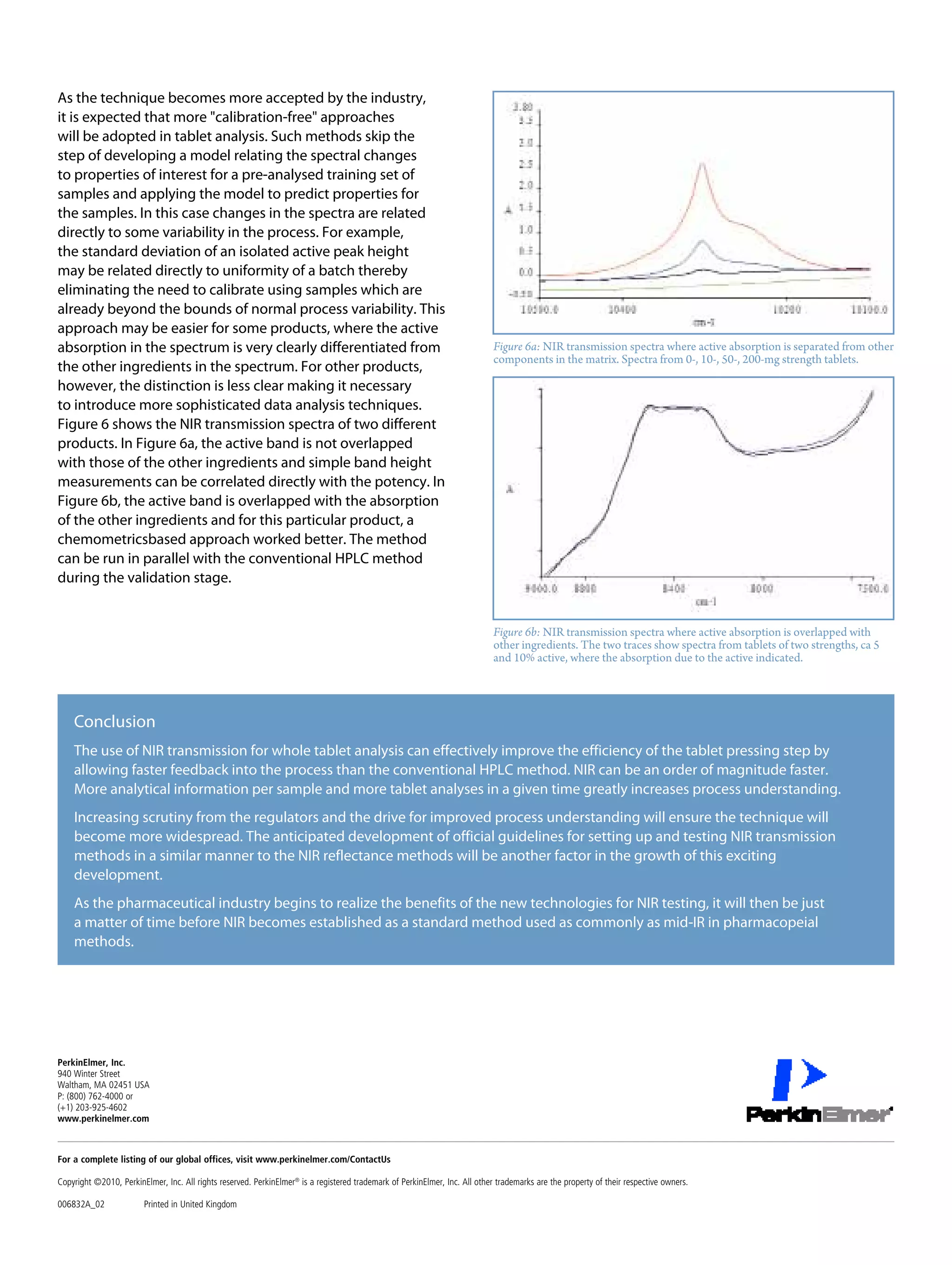
This document discusses the advancements in near-infrared (NIR) spectroscopy for whole tablet testing in the pharmaceutical industry, highlighting its potential to enhance measurement efficiency and process understanding compared to traditional HPLC methods. The method allows for non-destructive, rapid analysis of tablet content, which facilitates faster feedback during production and improves batch uniformity assessments. As regulatory scrutiny increases and technology improves, NIR is expected to become a standard testing method in pharmaceutical manufacturing.