The document outlines the role of pharmacovigilance (PV) in monitoring drug safety, detailing its establishment by WHO and the development of global databases for adverse drug reactions. It describes the operations of the Iraqi Pharmacovigilance Center (IPHVC), which is tasked with collecting and assessing drug safety information and making regulatory decisions based on reported adverse reactions. The document emphasizes the importance of reporting adverse drug reactions and the need for healthcare professionals to maintain vigilance to enhance patient safety.





















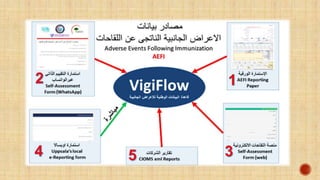
![ Reports are entered into the national database of ADRs and be analyzed
by expert reviewers regularly, so that validated ADRs reports be
uploaded to the global VigiBase® database, Uppsala Monitoring Center
(UMC).
Well - completed and duly submitted ADR reported by you may
result in IPhvC regulatory decisions, national alert-warning & various
harm prevention activities.
Report details’ are stored in a confidential database at the Iraqi
pharmacovigilance center [only & exclusively].
Before any information on a specific adverse drug reaction is utilized or
communicated, the identities of the patient, the reporter, and any other
medical professionals listed on a report will be omitted.
The ADR report will not be attributed to the reporter and will not imply
that the reporter or any other healthcare practitioner played a part in it in
any manner.](https://image.slidesharecdn.com/pharmacovigilance-240305220629-9209ba75/85/IQ-Pharmacovigilance-system-updated-pptx-23-320.jpg)



![ WORLD HEALTH ORGANIZATION WEBSITE, ESSENTIAL MEDICINES AND HEALTH
PRODUCTS. LINK:
HTTPS://WWW.WHO.INT/MEDICINES/AREAS/QUALITY_SAFETY/SAFETY_EFFICACY/PHARMVI
GI/EN/
IRAQI MINISTRY OF HEALTH, GUIDELINES FOR THE IRAQI PHARMACOVIGILANCE
SYSTEM (IPHVC) INDIV IDUAL CASE SAFTEY REPORT (ICSR) FOR HELTH CARE
PROFESSIONALS. 1ST EDITION 2012.
IRAQI MINISTRY OF HEALTH, DIRECTORATE OF TECHNICAL AFFAIRS, PHARMACY
DEPARTMENT, IRAQI PHARMACOVIGILANCE CENTRE. INDIVIDUAL CASE SAFTEY REPORT
IRAQI MINISTRY OF HEALTH, DIRECTORATE OF TECHNICAL AFFAIRS, PHARMACY
DEPARTMENT, IRAQI PHARMACOVIGILANCE CENTRE. DAILY RECORDS OF ADVERSE
REACTIONS RELATED TO DRUGS AGAINST COVID-19 REPORTS
IRAQI MINISTRY OF HEALTH, DIRECTORATE OF TECHNICAL AFFAIRS, PHARMACY
DEPARTMENT, IRAQI PHARMACOVIGILANCE CENTRE. ADVERSE EVENTS AFTER
IMMUNIZATION REPORTS
IRAQI MINISTRY OF HEALTH, DIRECTORATE OF TECHNICAL AFFAIRS, PHARMACY
DEPARTMENT, IRAQI PHARMACOVIGILANCE CENTRE. ADVERSE EVENTS FOLLOWING
IMMUNIZATION AEFI [PRESENTATION] 2021
IRAQI MINISTRY OF HEALTH, DIRECTORATE OF TECHNICAL AFFAIRS, PHARMACY
DEPARTMENT, IRAQI PHARMACOVIGILANCE CENTRE. IRAQI PHARMACOVIGILANCE
GUIDELINES FOR HEALTHCARE PROFESSIONALS. EDITION 2024](https://image.slidesharecdn.com/pharmacovigilance-240305220629-9209ba75/85/IQ-Pharmacovigilance-system-updated-pptx-27-320.jpg)