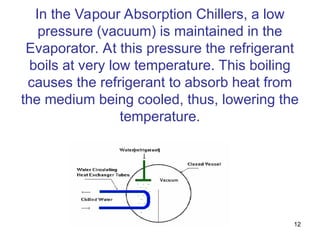
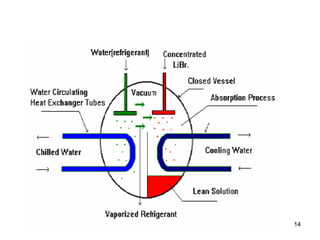
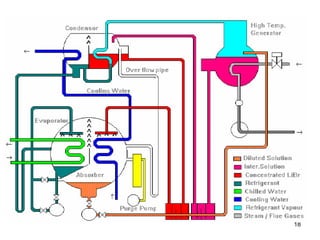
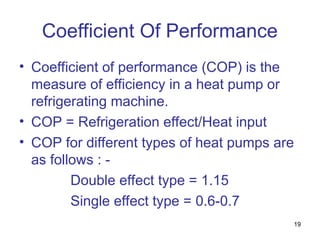
The document discusses the history and types of vapor absorption machines (VAM) invented in the late 19th century, highlighting both steam and direct-fired models. It explains the principles of refrigeration, the operation of VAM, and the technical components involved in the absorption process, including the roles of refrigerants and heat sources. Additionally, it addresses efficiency measures, factors affecting crystallization, and the use of inhibitors in maintenance.