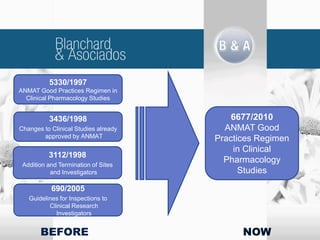
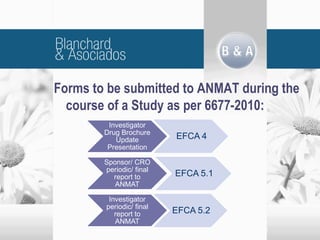
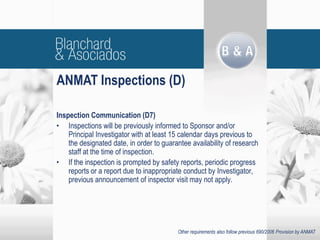
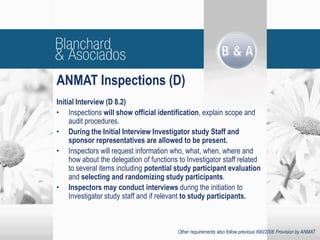
The document outlines new regulatory requirements from ANMAT, the regulatory body in Argentina, for clinical pharmacology studies, including additional documentation required for authorization requests, good clinical practice standards, inspections of investigators, and changes in scope and forms. Key changes include stricter documentation standards, additional safety reporting requirements, and expanded authority for ANMAT to inspect sponsors and CROs in addition to investigators.