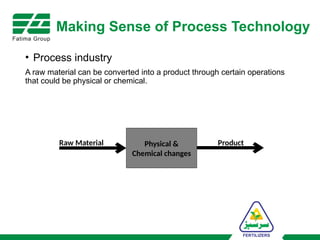
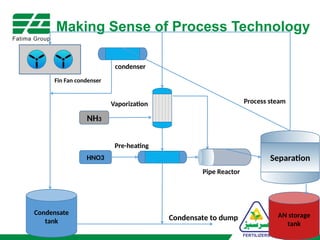
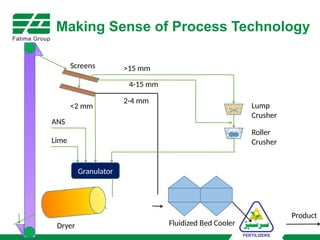
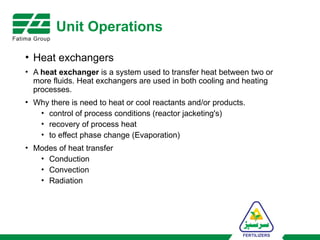
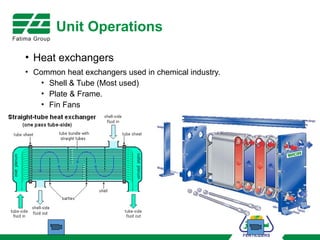
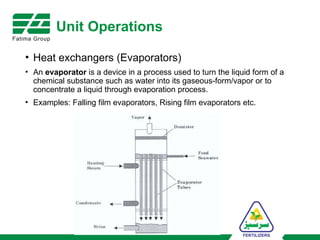
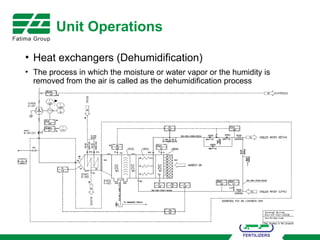
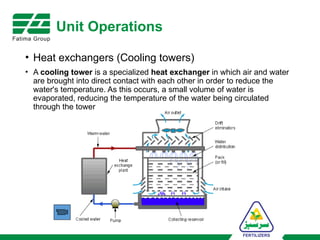
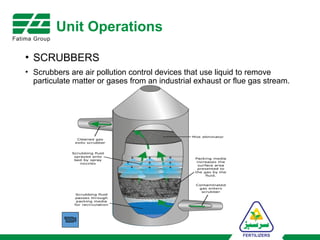
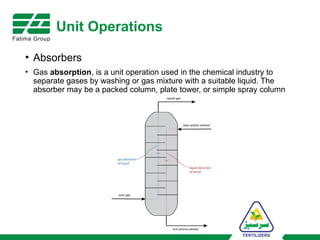
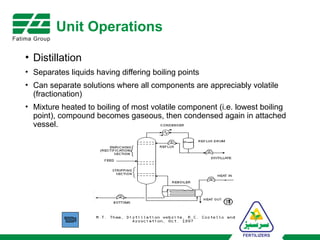
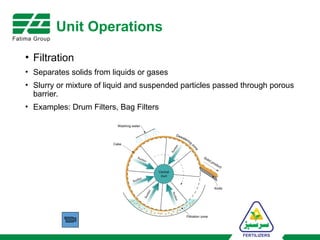
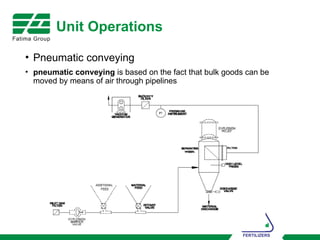
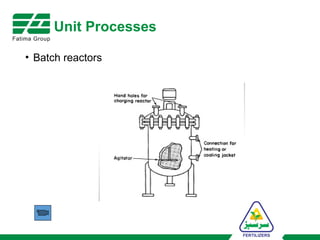
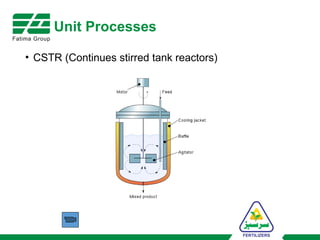
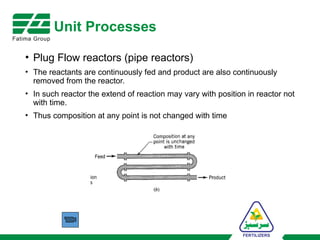
The document provides an overview of unit operations and unit processes within the chemical industry, distinguishing between physical changes and chemical reactions in processing technologies. It details various unit operations such as heat exchangers, evaporators, filters, and different types of reactors like batch and continuous reactors. Key concepts include methods of mass transfer, energy exchange, and separation techniques essential for chemical engineering.