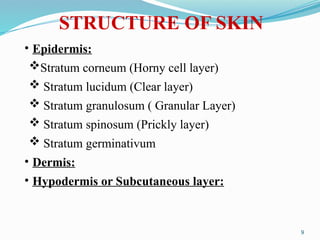
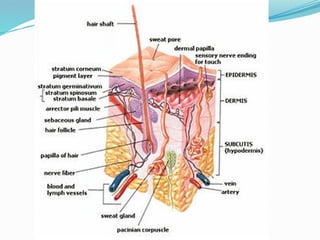
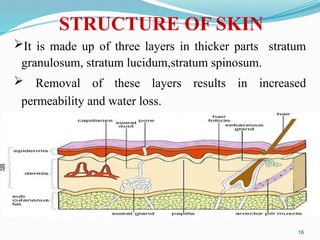
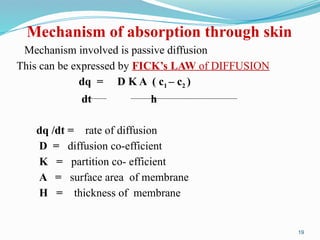
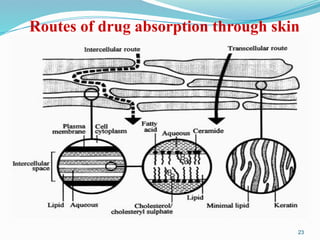
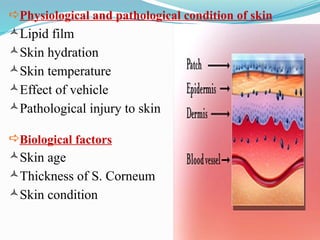
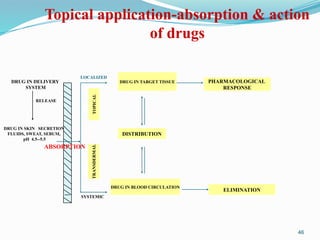
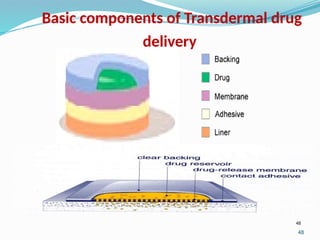
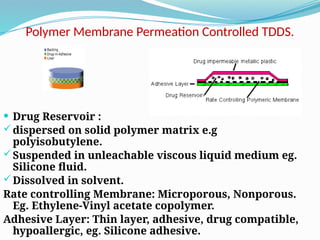
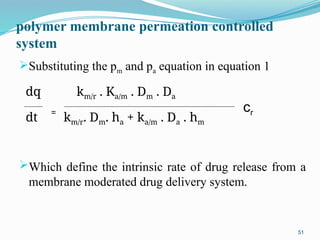
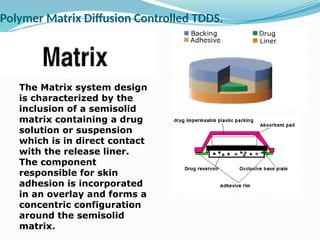
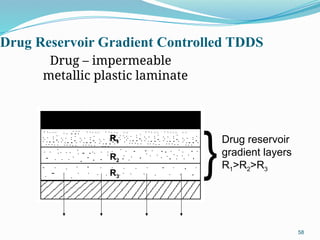
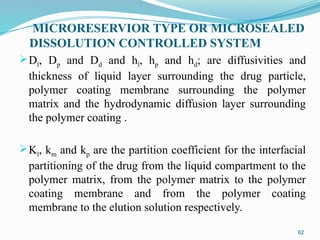
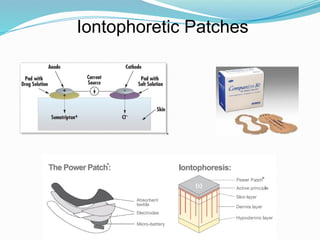
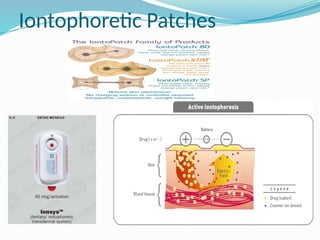
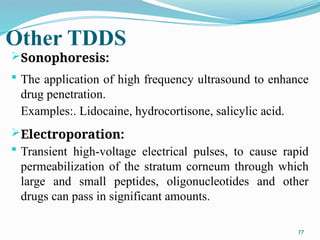
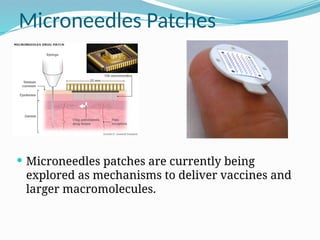
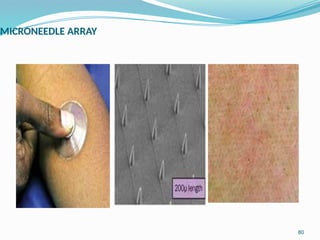
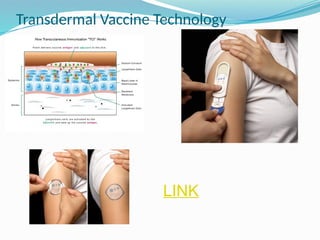
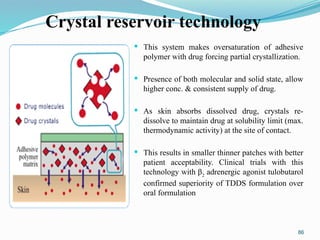
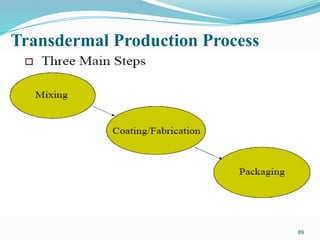
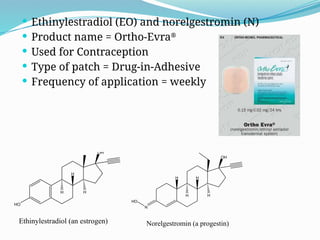
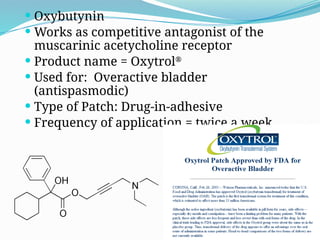
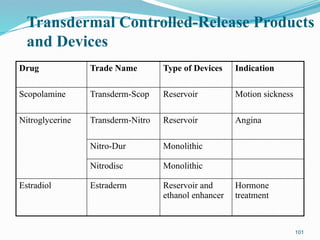
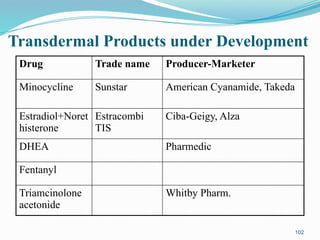
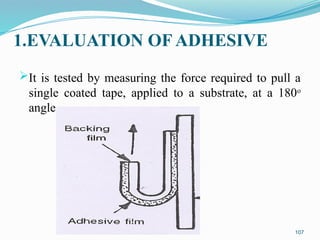
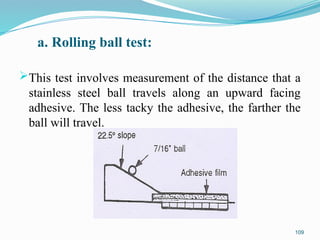
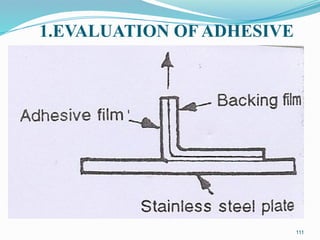
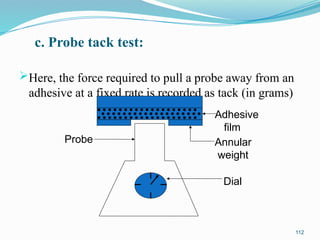
The document provides a comprehensive overview of transdermal drug delivery systems (TDDS), detailing their definition, history, advantages, and disadvantages. It discusses the mechanisms of drug absorption through the skin, factors affecting transdermal permeability, the components involved in TDDS formulation, and examples of successful transdermal patches. Additionally, it covers the technological approaches for developing TDDS, emphasizing the need for careful selection of drugs and polymers to ensure effective and safe delivery.