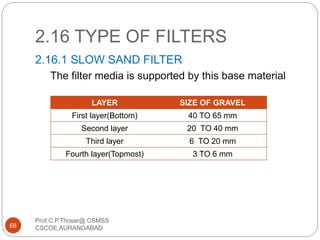
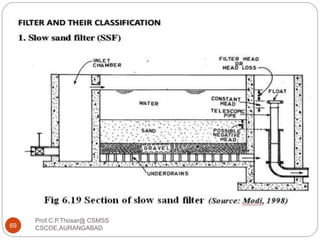
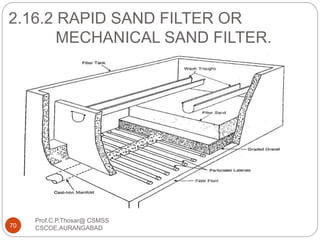
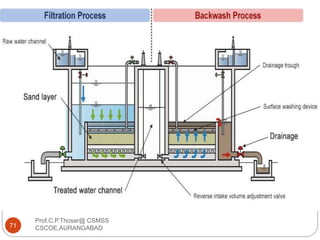
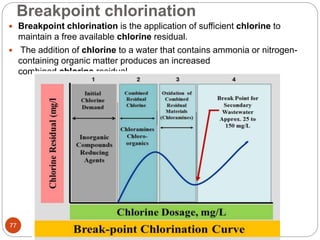
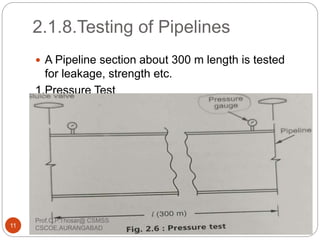
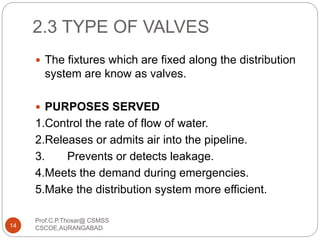
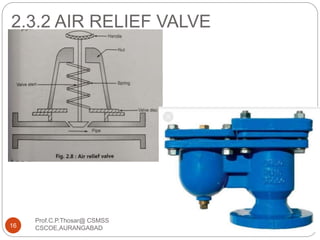
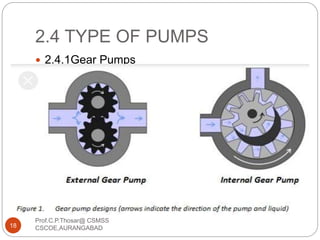
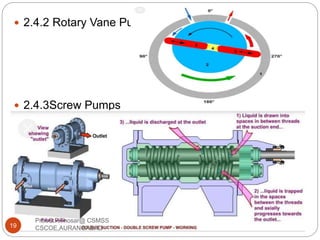
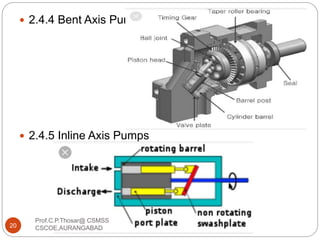
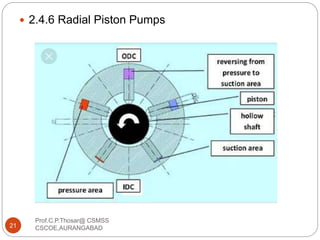
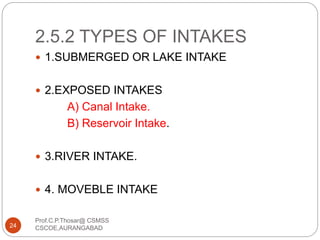
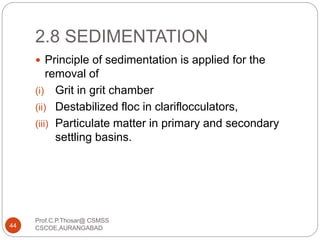
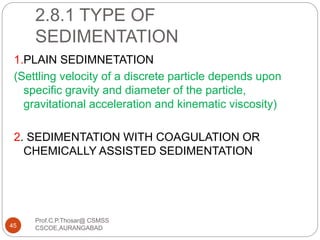
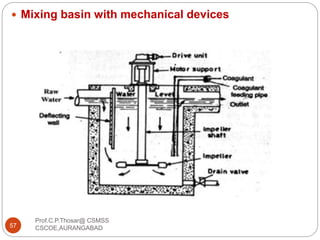
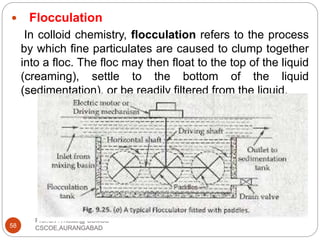
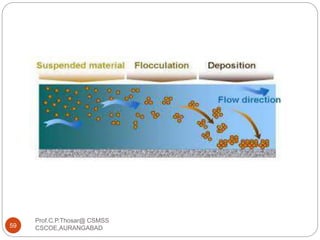
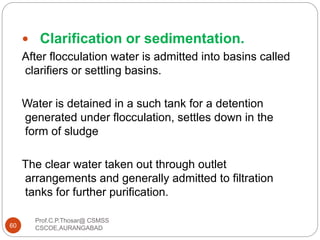
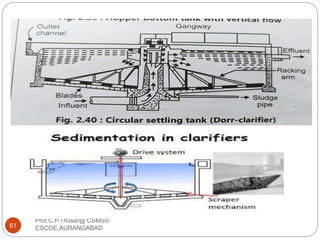
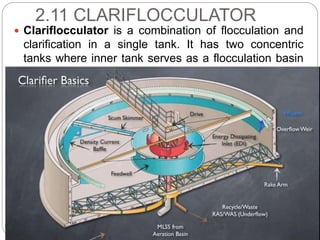
This document discusses the process of water treatment. It covers topics like conveyance of raw water through pipelines and canals, designing of rising mains, different types of valves and pumps used, intake structure design, and the various unit processes involved in water treatment - aeration, sedimentation, coagulation, flocculation, filtration, disinfection, and softening. The document provides detailed information on the working, design considerations, and examples of each treatment process.

























![2.15.2 FILTER MATERIALS
Sand
Anthracite
Anthracite, often referred to as hard coal, is a hard,
compact variety of coal that has a sub-metallic luster. It
has the highest carbon content, the fewest impurities, and
the highest energy density of all types of coal and is the
highest ranking of coals.
[ Carbon content (%): 92 – 98]
Garnet sand or limonite
Locally available materials
Gravel
67
Prof.C.P.Thosar@ CSMSS
CSCOE,AURANGABAD](https://image.slidesharecdn.com/unit-2-201106053608/85/Unit-2-67-320.jpg)