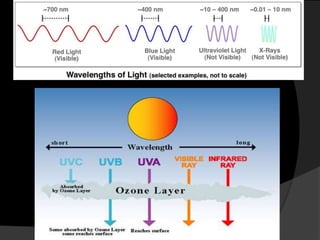
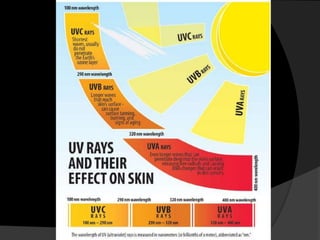
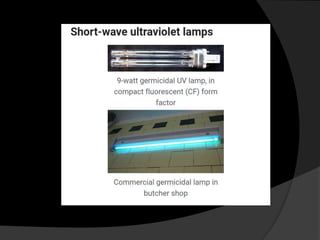
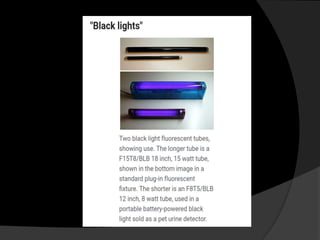
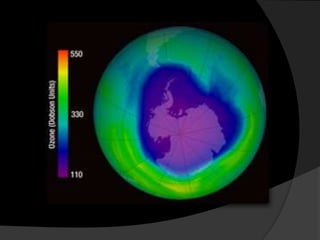
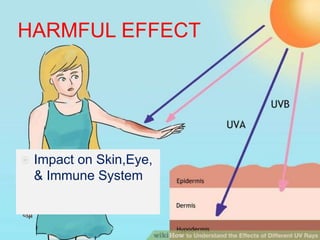
Ultraviolet (UV) radiation has wavelengths shorter than visible light. It is emitted by the sun and can be produced artificially. UV is divided into UVA, UVB, and UVC. While overexposure can harm health, moderate UV has benefits like vitamin D production. UV is used in applications like disinfection, lithography, and forensic analysis due to its ability to cause chemical reactions and fluorescence.