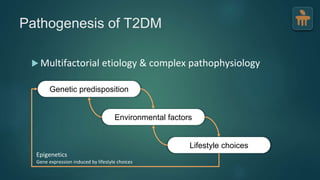
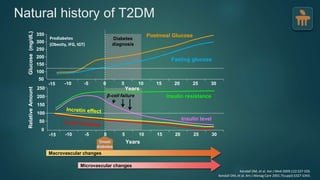
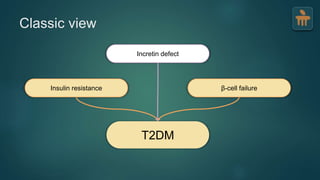
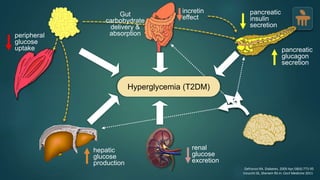
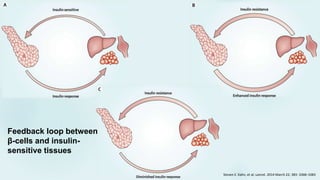
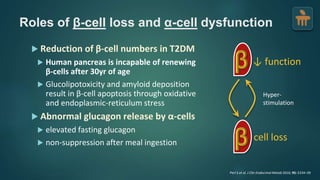
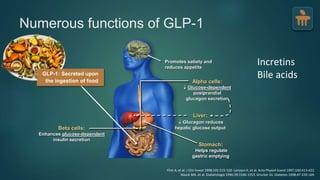
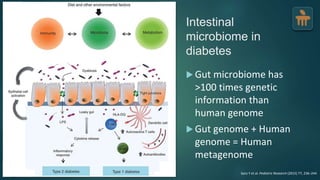
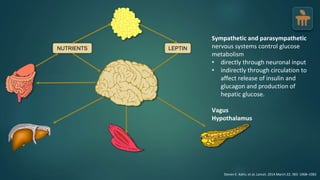
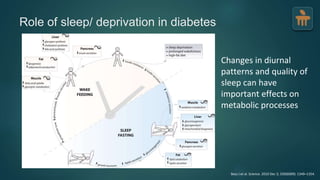
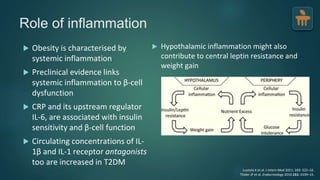
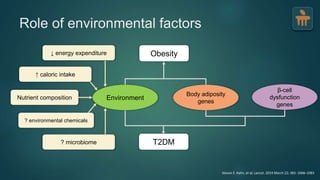
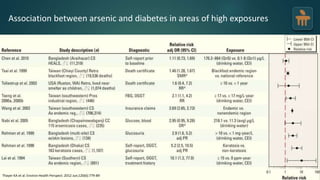
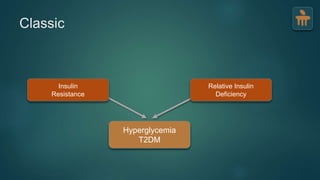
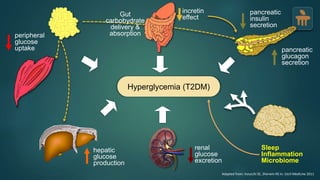
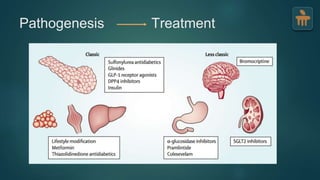
The document discusses the pathophysiology of type 2 diabetes mellitus (T2DM), highlighting its multifactorial causes including genetic predisposition, environmental factors, and lifestyle choices. It emphasizes the role of various bodily systems such as the gut microbiome, the brain, and inflammation in the disease's progression and management. The complex interactions between insulin resistance, β-cell dysfunction, and other metabolic changes underline the need for a comprehensive approach to treatment and prevention.