Embed presentation
Downloaded 61 times



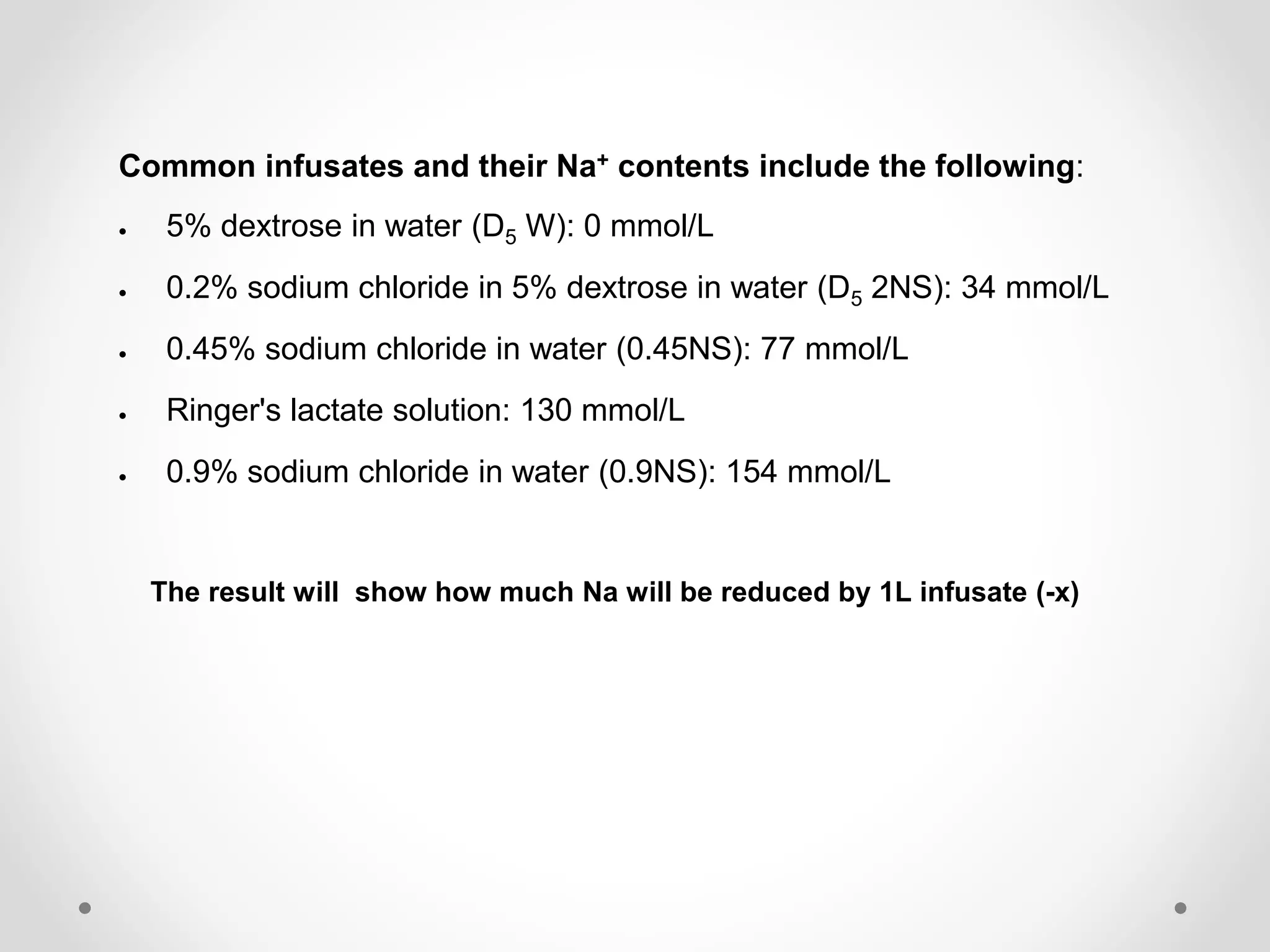



This document provides guidelines for treating hypernatremia. It outlines three equations for calculating water deficit, estimating the effect of intravenous fluids on serum sodium concentration, and determining the rate of fluid administration. Common intravenous fluids and their sodium contents are listed. The recommended approach is to use the equations to determine the fluid and rate needed to lower the patient's serum sodium by no more than 10 mmol/L over 24 hours, while also replacing insensible water losses.





