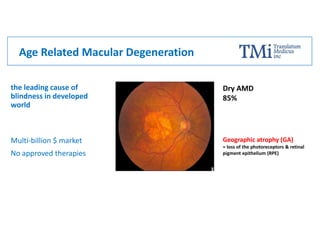
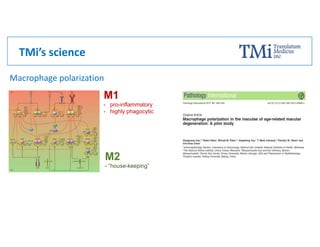
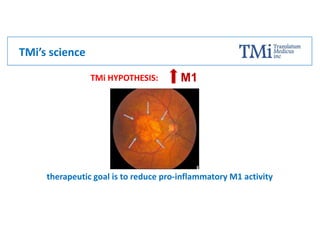
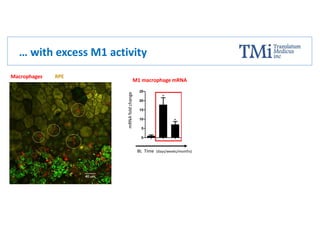
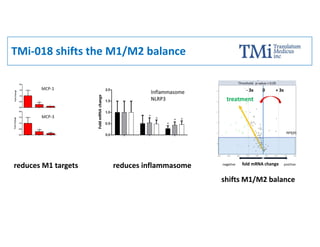
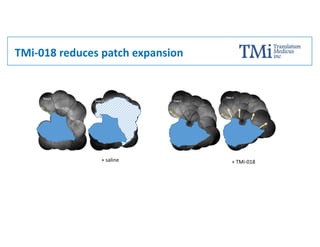
TMi has developed an animal model and lead compound that aims to de-risk and accelerate drug development for dry age-related macular degeneration (AMD). The animal model mimics dry AMD and has quantifiable regions of tissue loss analogous to geographic atrophy in humans. TMi's lead compound, TMi-018, is a first-in-class transcriptional regulator of M1 macrophages that has shown efficacy in reducing the onset and expansion of lesions in the animal model. TMi-018 has also demonstrated a clinically safe profile and potential for sustained release delivery required for treating dry AMD.