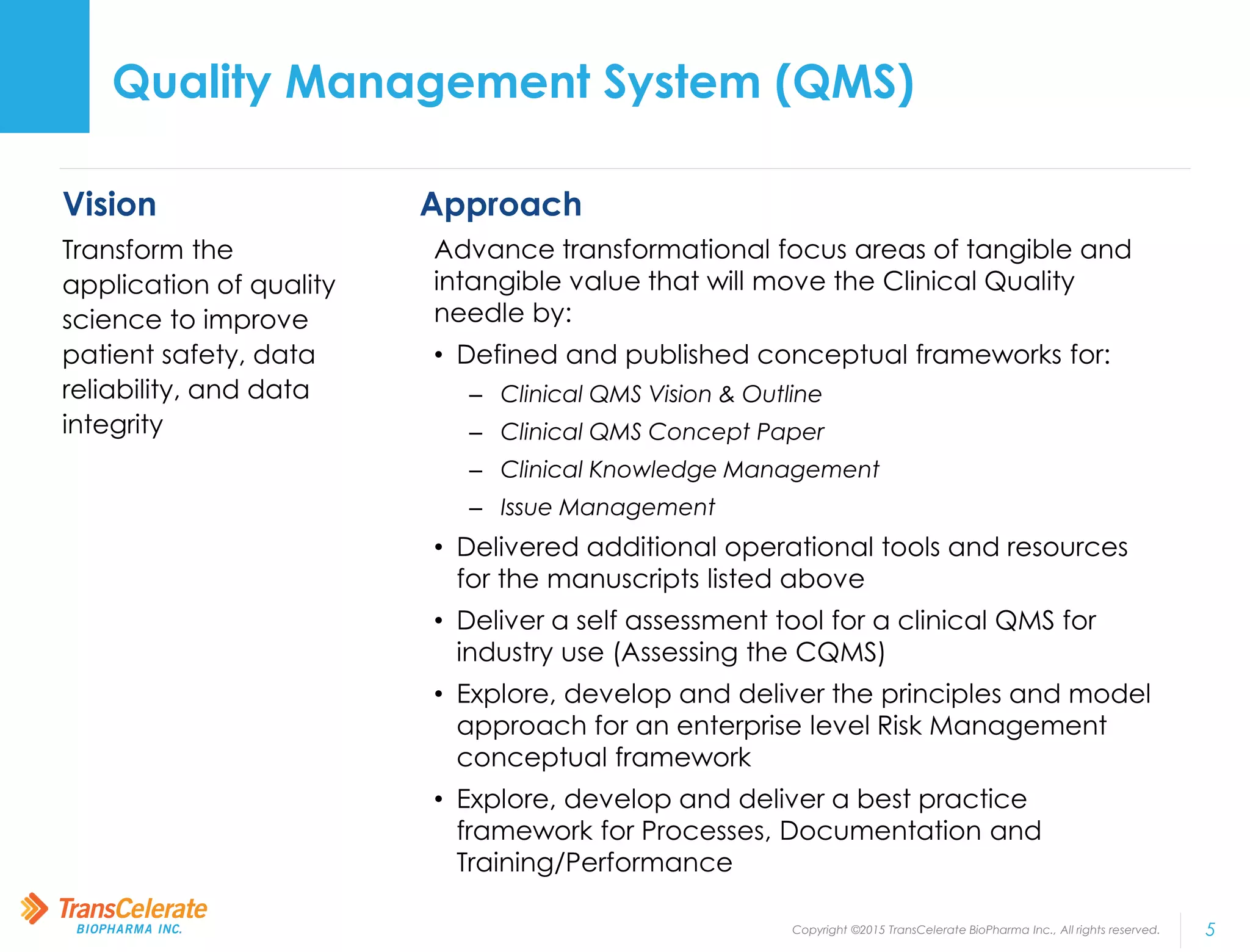
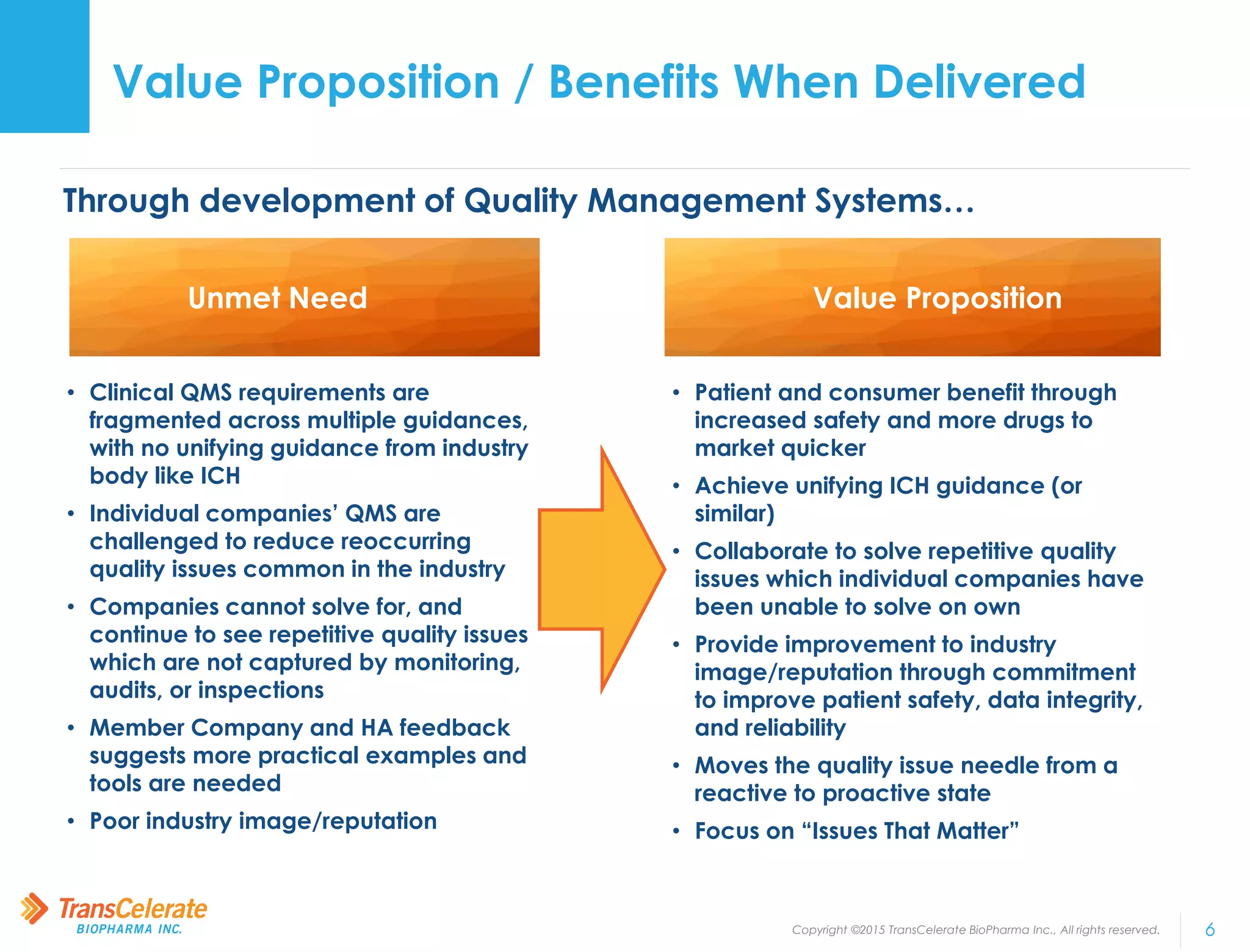
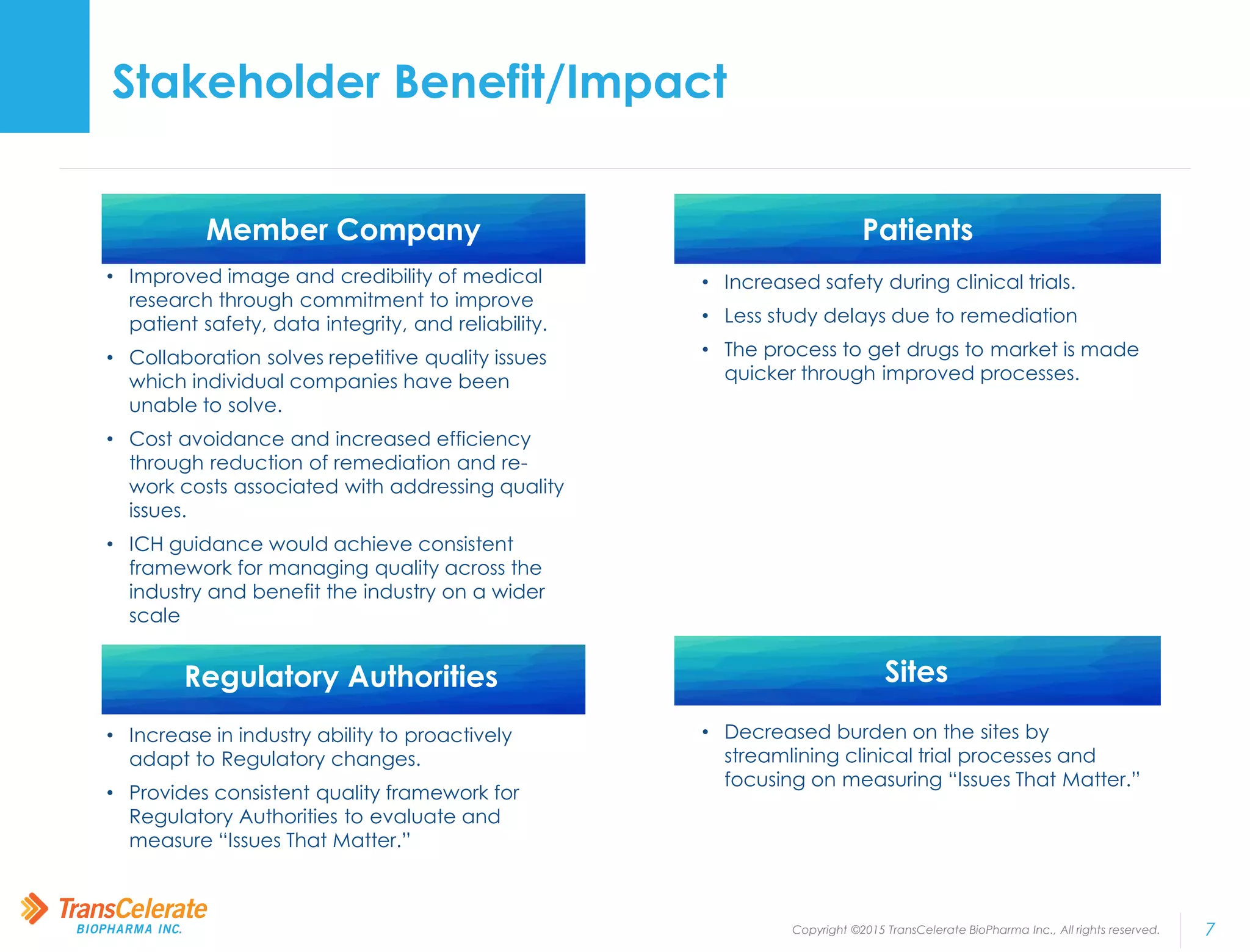
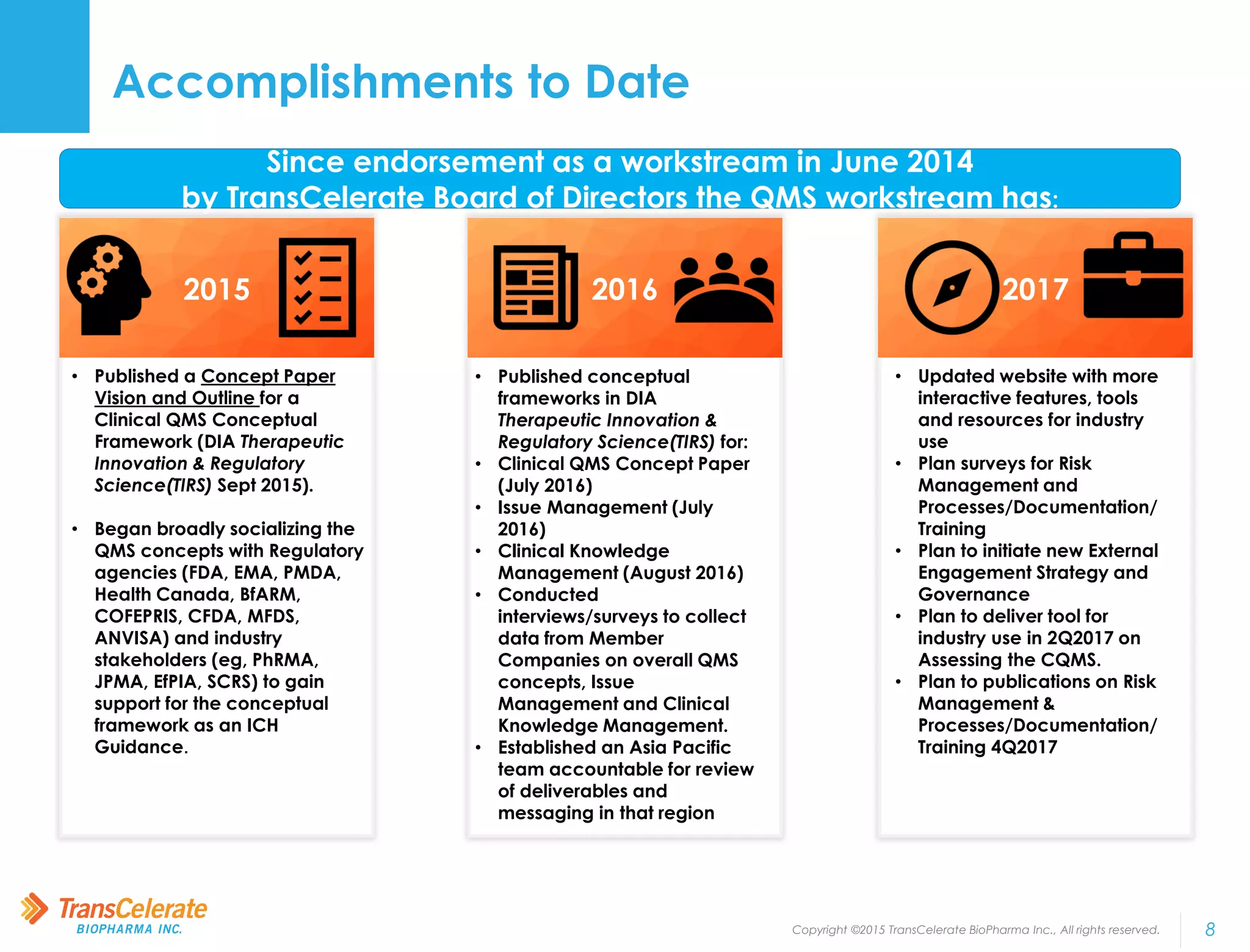
TransCelerate BioPharma is a non-profit organization whose mission is to collaborate across the biopharmaceutical industry to identify and implement solutions to improve the delivery of new medicines. The Quality Management System initiative aims to advance quality management across the industry by developing a conceptual framework for clinical quality management systems. The initiative has published several papers outlining its frameworks and tools. Its upcoming milestones include releasing additional tools and publishing additional framework papers on risk management and processes.