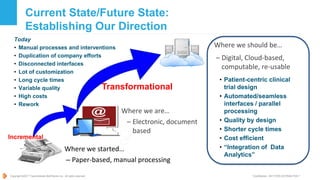
The document provides an executive summary of the Common Protocol Template (CPT) developed by TransCelerate BioPharma Inc. The CPT aims to streamline clinical trial protocols by standardizing format, content and terminology. This is intended to improve quality, reduce costs and complexity for sponsors, investigators, regulators and patients. The summary outlines the vision/mission of TransCelerate, the rationale for developing a common template, its near term and potential future benefits for stakeholders, guidance principles, and alignment of the CPT with the NIH-FDA clinical trial protocol template.




![Copyright ©2017 TransCelerate BioPharma Inc., All rights reserved. * Confidential - NOT FOR DISTRIBUTION *
• A common protocol template structure with
harmonized language
• Streamlined content enables identification of
critical information for end users
• Begin working towards model endpoint
definitions to align with Clinical Data Interchange
Standards Consortium (CDISC) Therapeutic
Area (TA) data standards. Asthma and Diabetes
available in the first release.
• Reconnect processes (protocol, eCRF,
development)
• Transformation of the design process
o Analytics-driven trial design, modelling, scenario
planning
• Role-based access to protocol
information (Principal Investigator [PI], Ethics,
Participants)
• Connection to other systems
Common Protocol Template is Intended to Prepare
for the Future State
Human-
Readable
Protocol
IRB/IECs
Sites
Regulators
Foundation
Machine-
Readable
Protocol
Metadata driven
processes
Content Reuse
Disclosure
SAP
CSR
eCRF
Statistical Output
Future](https://image.slidesharecdn.com/cptimpltk-exec-summaryv003-180104173718/85/Common-Protocol-Template-CPT-Initiative-Implementation-Toolkit-Executive-Summary-6-320.jpg)