Embed presentation
Download to read offline



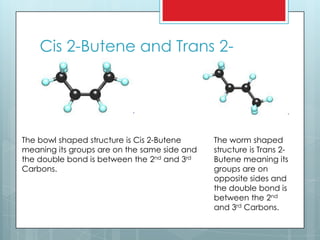


Trans 2-butene is an isomer of butene that has four carbons and eight hydrogens. It has a trans configuration, meaning the groups on either side of the double bond are on opposite sides. The document contrasts the cis and trans isomers, showing the bowl shape of cis 2-butene and worm shape of trans 2-butene. Fun facts provided include that butene is used to form ground-level ozone and is both smelly and flammable, despite being colorless.





