Embed presentation
Download to read offline



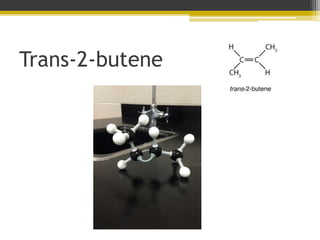


There are two geometric isomers of 2-butene called cis-2-butene and trans-2-butene that differ only in whether the fourth carbon is on the same side or opposite side of the third carbon. While they have the same chemical formula of C4H8 and similar boiling points, they are different structures due to the inability of double bonds to rotate like single bonds can.





