Embed presentation
Download to read offline

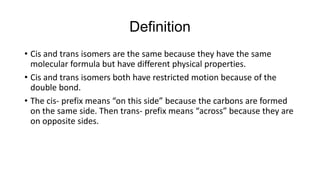

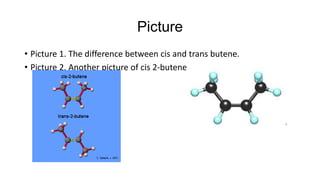


Cis-2-butene is a geometric isomer of butene where the carbons are on the same side of the double bond. Cis and trans isomers have the same molecular formula but different physical properties due to restricted motion from the double bond and differing dipole moments. Cis-2-butene specifically has all carbons on one side of the double bond, as opposed to across from each other in trans isomers.





